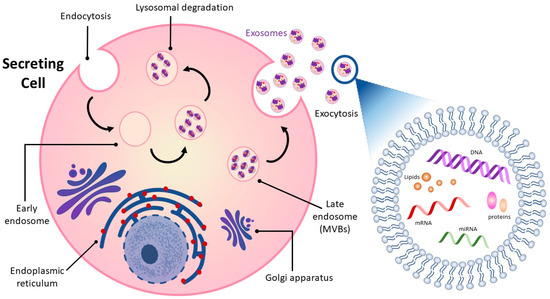
2. Overview of Exosomes
Intercellular communication among cells is vital in all living organisms for normal functioning [
13,
14]. Cell-to-cell communication occurs through direct contact in neighboring cells or via secretion of soluble factors, such as cytokines, hormones, and chemokines, which can extend interaction over distances [
13,
15]. Recent attention has focused on a means of intercellular communication via extracellular vesicles (EVs), cell-derived membrane-enclosed microvesicles of varying sizes secreted by most cell types [
16,
17]. EVs show promise in a number of medical applications and may be useful as non-invasive diagnostic biomarkers and therapeutic nanocarriers for the treatment of various diseases, including neurodegeneration, cardiovascular dysfunction, and cancer [
18,
19,
20,
21]. EVs can be categorized into four major classes, microvesicles, apoptotic bodies, virus-like particles, and exosomes, according to their size, subcellular origin, and content [
22,
23]. While the first three classes of EVs are formed by the outward budding of the plasma membrane, exosomes originate as intraluminal vesicles contained within multi-vesicular bodies (MVBs) (
Figure 1) [
24,
25]. Successive extracellular secretion of these intraluminal vesicles by fusion with the plasma membrane releases exosomes with diameters of ~40 to 160 nanometers into the extracellular matrix [
12,
20,
26].
Exosomes released into surrounding bio-fluids mediate cell–cell communication between adjoining and distant cells by shuttling cell-specific cargo composed of bioactive molecules such as lipids, carbohydrates, metabolites, surface and cytoplasmic proteins, and nucleic acids [
27,
28]. Exosome cargo is subject to selective sorting, is unique to the cell type from which they are derived and reflects the functions and current state of the cell of origin [
29,
30]. Exosomes are also enriched in specific proteins such as tetraspanins that distinguish them from their cell of origin and can be used to track and identify them [
31]. Their lipid bilayer structure is well-suited to carrying and protecting their contents over time and distance so that they can be delivered intact from donor to receptor cell and from one part of the organism to the other.
3. The Link between Cardiovascular Disease and Exosomes
3.1. Exosome Cargo and the Cardiovascular System
Exosomes are key factors in regulating CVD progression due to their role in the transport and exchange of signaling molecules [
32,
33,
34,
35]. A study by Peng et al. found that extracellular vesicles from atherosclerotic plaque with characteristics of exosomes may be able to spread atherosclerosis distally [
36]. Extracellular vesicles were isolated from carotid atherosclerotic lesions of rats with knockout of the LDL receptor gene on a high-fat diet and injected into LDL receptor knockout mice on a normal diet, conditions under which they do not normally develop atherosclerosis. The extracellular vesicles from atherosclerotic tissue were taken up by endothelium in the carotids and promoted endothelial inflammation.
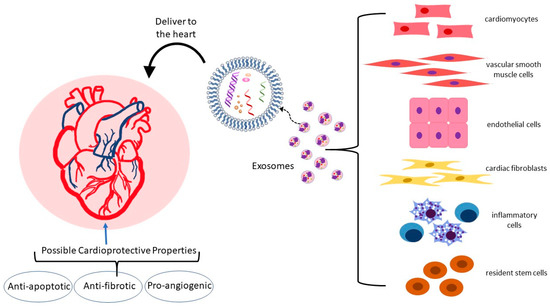
In the heart and vasculature, cell types that interact to maintain homeostasis and are known to release exosomes include cardiomyocytes, endothelial and vascular smooth muscle cells, cardiac fibroblasts, inflammatory cells, and resident stem cells (
Figure 2) [
35,
37,
38,
39]. Under both physiological and pathological conditions, exosomes are an integral mode of communication amongst these cell types (
Table 1). Their rate of secretion and specific miRNA cargo can change in response to pathological states or drug treatments [
40,
41,
42,
43]. For example, in a mouse model, analysis of the miRNA content of exosomes derived from EPC found that the top ten miRNAs in abundance were all associated with atherosclerosis [
44]. Administration of EPC-derived exosomes to atherosclerosis-prone hyperglycemic mice lowered oxidative stress and inflammation and reduced plaque area, indicating that EPC exosomes may improve endothelial function in diabetic atherosclerosis.
Further supporting exosome influence on atherosclerosis of specific exosome cargo from distinct cell types, cardiomyocyte exosomes are enriched for heat shock proteins (HSP) such as HSP-20, 60, and 70) [
45,
46,
47]. HSPs are molecular chaperones that aid in protein folding and take part in apoptotic processes, cell proliferation, and inflammatory responses [
48]. A higher expression of HSP-20 has cardioprotective effects, likely via the inhibition of TNF-α and IL-1β [
46,
49]. HSP-60, localized within mitochondria, supports mitochondrial function and protein homeostasis under stress conditions, but when released extracellularly, is apoptotic, inflammatory, and is considered destructive and atherogenic [
50,
51]. HSP-70 is found on the exosome surface and, by binding to toll-like receptor-4 on the cardiomyocyte, it can activate the MAPK/ERK1/2 signaling pathway leading to pro-survival and cytoprotective effects [
52,
53].
Exosomal HSP-27 release can also affect macrophage cholesterol homeostasis [
54,
55]. HSP-27 is considered atheroprotective, and low levels of this protein in plasma are associated with CVD [
56]. Shi et al. combined HSP-27 with an anti-HSP-27 antibody to form immune complexes and found that exposure of cholesterol-loaded THP-1 human macrophages to these immune complexes augmented release of exosomes and these exosomes were enriched in cholesterol, thus facilitating macrophage cholesterol efflux [
57]. They propose the possibility of packaging HSP-27 immune complexes in exosomes as a means of delivering immune therapy against atherosclerosis to macrophages.
Exosomes can also carry lipids, including free fatty acids, which Barcia et al. showed can be delivered to the heart in vivo and to cardiac cell types in vitro [
58,
59]. This group isolated serum exosomes from healthy adults under fasting conditions and after consumption of a high-calorie meal (post-prandial) and found that each could take up a free fatty acid analogue, but the post-prandial exosomes had higher levels of the scavenger receptor CD36 content and higher lipid content. Blocking CD36 reduced free fatty acid analogue uptake, indicating an active role for this receptor in exosomes. In cell culture experiments, cardiac endothelial cells and cardiomyocytes were able to take up the free fatty acid analogue from serum exosomes and the mouse heart was able to take up the analogue after tail vein injection of the exosomes, leading the authors to posit a role for exosomes in delivering this type of lipid fuel to the heart.
3.2. Exosomes in Atherosclerosis: Endothelial Dysfunction, Macrophage Recruitment, and Vascular Smooth Muscle Behavior
Endothelial dysfunction is an early step in the development of atherosclerosis. Activated endothelial cells upregulate expression of adhesion molecules ICAM-1, VCAM-1, P-selectin, and E-selectin, which act to recruit monocytes to the subendothelial layer of the artery. Important risk factors for atherosclerosis that cause activation of endothelium are an elevation in serum levels of oxidized LDL (ox-LDL) and an inflammatory environment of elevated cytokines and homocysteine [
60,
61,
62,
63]. Ox-LDL and homocysteine have been shown to induce the release of HSP-70-containing exosomes from aortic endothelial cells that can elicit immune inflammatory responses and selectively activate monocytes [
64,
65]. Monocyte activation leads to monocyte-endothelial cell adhesion and monocyte infiltration into the subendothelial space. Activated monocyte-derived macrophages in the arterial intima orchestrate multiple inflammatory atherosclerosis-promoting processes in plaque formation [
66]. When activated, monocytes themselves can perpetuate their own adhesion to endothelium by producing exosomes that enter endothelial cells and activate NF-κB, thus causing endothelial production of adhesion molecules [
67]. Exosomes isolated from murine bone marrow-derived macrophages (BMDM) and infused into atherosclerosis-prone ApoE-deficient Western diet-fed mice exhibit an ability to reduce atherosclerotic lesion necrosis and stabilize atheroma [
68]. Cheng et al. showed, in a human cell culture model, that human umbilical vein endothelial cells cultured under inflammatory conditions were protected from apoptosis by exosomes from M2 polarized THP-1 macrophages and the effect was mediated by miR-221-3p [
69].
VSMC proliferation, migration, and cytokine secretion contribute to atherogenesis [
70]. VSMC behavior can be influenced by exosomes of macrophage origin. As far back as 2016, Niu et al. showed that extracellular vesicles derived from J774a.1 macrophage foam cells could enhance migration and adhesion of human aortic VSMC [
71]. Ren et al. cultured VSMC in media with exosomes from ox-LDL-stimulated macrophages and found that these exosomes improved viability and invasive properties of the VSMC while suppressing apoptosis [
72]. Of the overexpressed miRNA in the ox-LDL-stimulated macrophage exosomes, miR-186-5p was found to be responsible for these pro-atherogenic effects via inactivation of SHIP2, thus enhancing PI3K/AKT/mTOR signaling.
The influence of VSMC-derived exosomes on atherosclerosis has been studied. Exosomes isolated from cultured primary human VSMC induced to undergo premature senescence and their properties were then analyzed [
73]. The senescent VSMC produced more exosomes than the control VSMC. When T cells and monocytes were exposed to exosomes of senescent VSMC origin, they produced more pro-inflammatory cytokines than cells exposed to control VSMC exosomes.
3.3. Exosomes in Apoptosis
A critical event in CVD progression, including myocardial infarction and heart failure, is the apoptosis and autophagy of cardiomyocytes [
74]. Myocardial ischemia followed by therapeutic restoration of blood flow (ischemia-reperfusion) leads to cardiomyocyte damage and apoptosis in both the hypoxia stage and the rapid recovery stage [
75,
76]. Cardiac fibroblasts release exosomes that rescue cardiomyocytes from ischemia-reperfusion injury by protecting against apoptosis and pyroptosis (a form of programmed necrosis) [
52,
77,
78]. Luo et al. showed that injection of rat cardiac fibroblast exosomes into the infarct border of the rat heart during hypoxia–reoxygenation (left anterior descending artery ligation and reperfusion) reduced infarct size. Comparison of expression levels of exosomal miRNAs showed miR-423-3p to be enriched in exosomes that limited infarct size and knockdown of this miRNA in cell culture companion studies confirmed its importance in maintaining cell viability and reducing apoptosis [
79].
In studies using human exosomes, Qiao et al. isolated exosomes from conditioned media of cardiac cells from healthy volunteers and heart failure patients and performed intramyocardial injection of these exosomes into a mouse model of acute myocardial infarction induced by coronary vessel ligation. The study showed that the healthy volunteer exosomes had favorable effect on healing, apoptosis, and tissue preservation while exosomes of heart failure patients inhibited cardiomyocyte proliferation, inhibited angiogenesis, and worsened healing [
80]. As in the case of exosomes from heart failure patients, exosomes from EPC of mice deficient in the anti-inflammatory and atheroprotective cytokine IL-10 also lose their protective effect in reducing infarct damage and, rather than inhibit apoptosis, these exosomes enhanced apoptosis [
81,
82].
3.4. Exosomes in Hypertrophy
Pathologic cardiac hypertrophy occurs when the wall of the ventricle thickens, impairing systolic function [
83]. It is often a result of chronic hypertension and can lead to ischemia and eventually to heart failure. Hallmarks of this process are enlargement of cardiomyocytes and fibrosis. Exosomes have been implicated in the regulation of cardiac hypertrophy via effects on the renin-angiotensin system. The mechanical stretch of cardiomyocytes has been found to release exosomes enriched with angiotensin II and its receptor content, contributing to increased vascular resistance and hypertrophy [
84,
85,
86].
Constantin et al. found a potential benefit of exosomes from human mesenchymal stem cells in ameliorating hypertrophy in a model of human hypertrophic cardiomyocytes [
87]. When hypertrophic cardiomyocytes were incubated with extracellular vesicles from subcutaneous adipose tissue stem cells or from bone marrow mesenchymal stem cells, cardiac markers for cardiomyocyte hypertrophy and inflammatory cytokine such as IL-1β, IL-4, IL-6, and TNF-α decreased [
88].
Mao et al. engineered exosomes derived from human cardiosphere-derived cells to target cardiomyocytes [
89]. They then infused these exosomes into a mouse model of myocardial hypertrophy and showed decreased perivascular and myocardial interstitial fibrosis of the left ventricle and decreased hypertrophy in treated mice versus sham mice and attributed the differences largely to effects of miRNA-148a, an miRNA with anti-proliferative properties.
Ren et al.
[1] evaluated the effect of bone marrow mesenchymal stem cell-derived exosomes from Sprague-Dawley rats on cultured H9c2 cells (rat embryonic cardiomyocytes) treated with angiotensin (Ang) II to induce hypertrophy and found that the exosomes increased viability of the Ang II-treated H9c2 cells while reducing apoptosis and inflammation. The bone marrow mesenchymal stem cell-derived exosomes acted, at least in part, by normalizing expression of Hippo-Yes-associated protein (YAP) signaling pathway proteins that had been altered by Ang II.
3.5. Exosomes in Angiogenesis
The regulation of blood vessels involved in supporting the myocardium with oxygen and nutrients relies, in part, on communication between cardiomyocytes and myocardial vascular endothelial cells via exosomes [
90]. Angiogenic capacity is of great importance in cardiac repair and regeneration post-ischemic injury. A number of cell types can release exosomes that deliver specific pro-angiogenic miRNAs. For example, mesenchymal stem cell exosomes isolated from human adipose tissue have been shown to promote angiogenesis in human umbilical vein endothelial cells by carrying miR-125a [
91]. Exosomes extracted from cardiomyocytes after cardiomyocyte hypoxic preconditioning can protect cardiac microvascular endothelial cells from oxidative damage and promote angiogenesis in vitro and in vivo and this effect is mediated predominantly by miR-222 and miR-143 [
92,
93]. Gou et al. showed that purified exosomes from primary neonatal cardiomyocytes treated with H2O2 to induce injury were enriched in miR-19a-3p and this increase in miR-19a-3p was also true for human plasma in post-myocardial infarction patients [
94]. They demonstrated uptake of exosomes from mouse neonatal cardiomyocytes by endothelial cells and showed that miR-19a-3p was anti-angiogenic and that neutralizing this miRNA promoted survival and proliferation of endothelial cells. They performed mouse studies in which they induced myocardial infarction via ligation of the left anterior descending coronary artery and then silencing of miR-19a-3p and showed that silencing improved angiogenesis. The target of miR-19a-3p was found to be hypoxia-inducible factor (HIF)-1α, which is known to drive multiple genes involved in angiogenesis [
95]. In a rat model of myocardial infarction induced by ligation of the left anterior descending coronary artery, exosomes derived from cardiac telocytes enhanced angiogenesis and reduced cardiac fibrosis [
96]. Increased miR-126 and miR-199a in circulating exosomes in patients with coronary artery disease is associated with a reduced risk for major future adverse cardiovascular events [
97]. Cultured endothelial cells exposed to these exosomes showed increased proliferation, migration, and tube-formation [
98,
99].
In the microenvironment induced by myocardial infarction, M1 macrophages predominate early, are inflammatory, and generate exosomes that transport miRNAs to endothelial cells that can reduce angiogenic potential and aggravate myocardial injury [
100]. These proinflammatory exosomes are enriched in miR-155, which is known to inhibit pathways within endothelial cells, such as the Sirtuin 1, adenosine monophosphate-activated protein kinase (AMPK), and endothelial nitric oxide synthase which promote angiogenesis [
101,
102].
3.6. Exosomes in Cardiac Fibrosis
Cardiac fibroblasts play an integral role in normal myocardial structure and physiology [
103]. They produce an extracellular matrix and pro-fibrotic factors that can be reparative following injury [
104]. However, when pathologically activated, they negatively affect cardiac function as well as myocardial compliance and stiffness [
105,
106,
107,
108]. Exosomes derived from different cell types and originating under normal or pathologic conditions can affect fibroblasts and modulate cardiac fibrosis. For instance, in a mouse model of diabetes, exosomes released from the heart after exercise on a treadmill reduced fibrosis via their higher content of miR-29b and miR-455, which acted by downregulating pro-fibrotic matrix metalloproteinase (MMP)-9 [
109]. In humans with heart failure, the level of miR-217 in cardiac tissue is elevated and higher miR-217 correlates with poorer left ventricular ejection fraction [
110,
111]. In a mouse model of heart failure induced by thoracic aortic constriction, cardiomyocyte-derived exosomes enriched in miR-217 aggravated cardiac fibrosis via targeting of phosphatase and tensin homolog (PTEN) [
111]. Yuan et al. also used the thoracic aortic constriction mouse model to look at cardiac remodeling under mechanical stress and found inhibition of excessive fibrosis in mice given an miR-378 mimic. Exosomes from cultured cardiomyocytes that had been subjected to mechanical stress harbored miR-378 and when cardiac fibroblasts that had also been subjected to mechanical stress were exposed to these exosomes, they showed reduced upregulation of collagen expression and lower MMP9 protein levels [
112]. Anti-fibrotic effects on the cardiac fibroblasts were blocked with depletion of miR-378 from the stretched cardiomyocyte conditioned medium. Using murine cells in culture, Tang et al. showed that mechanical stress-treated cardiomyocytes can activate cardiac fibroblasts by delivering miR-494-3p in exosomes [
113]. The cardiomyocyte exosomal miR-494-3p targets PTEN in the fibroblasts. The miR-494-3p enrichment of cardiomyocyte exosomes occurs due to stress-induced upregulation of the E3 ubiquitin ligase Peli1, a known participant in inflammatory processes, and is abrogated in cardiomyocytes that are deficient in Peli1.
The pathological activation of cardiac fibroblasts is also influenced by exosomes derived from macrophages. In the diabetic microenvironment, exosomes released from macrophages exacerbate cardiac fibrosis [
114].
3.7. Exosomes in Myocardial Infarction
Exosomes are considered potential biomarkers for the diagnosis and monitoring of myocardial ischemia and infarction [
115,
116]. This is of value because of the limitations in sensitivity and specificity and delay in detectable change in the standard marker, troponin T [
117]. Earlier diagnosis and confirmation of myocardial infarction allows faster intervention for heart muscle preservation [
118].
Silvia-Palacios conducted a study in Mexico City in which exosomes were isolated from the plasma of 26 patients admitted to intensive care with acute ST-segment elevation myocardial infarct (STEMI) and compared to those of 26 healthy donors [
119]. They found that ischemia prompted increased exosome release from the myocardial infarction patients. Both during the acute myocardial infarction and after reperfusion, miR-223-3p, an miRNA that targets inflammatory molecules, was found at high levels in patient exosomes.
Chen et al. looked at differentially expressed miRNAs in plasma exosomes from patients with STEMI and non-STEMI versus healthy controls and proposed a set of 10 miRNAs that they found could discriminate between STEMI and non-STEMI, which has implications for prognosis and treatment planning [
120].
3.8. Adipose Tissue Exosomes
Obesity is often associated with atherosclerosis and the metabolic complications of obesity may implicate adipose-tissue (AT)-derived exosomes, although their explicit role in atherogenesis remains unclear [
121,
122]. Adipocyte-derived exosomes have been shown to deliver adipocyte-dominant transcripts into macrophages and promote AT macrophage activation [
123,
124]. Xie et al. showed that exosomes released from differently located and stressed ATs have distinct pro-atherosclerotic effects [
121]. Barberio et al. characterized miRNAs from human adipose tissue-derived exosomes from obese and lean adolescents and found that six miRNAs contained in adipocyte exosomes targeted cholesterol efflux genes and were significantly associated with cholesterol efflux capacity [
125]. They then exposed cultured THP-1 macrophages to adipocyte-derived exosomes and showed that obese subject exosomes significantly increased macrophage ox-LDL retention compared to lean subject exosomes. The findings of this study exemplify a mechanistic link between obesity and macrophage lipid handling.
Liu et al. showed, in mice, that perivascular adipose tissue exosomes suppress macrophage foam cell transformation [
126]. Treatment of a mouse macrophage cell line with the perivascular adipose tissue exosomes reduced ox-LDL uptake, likely due to decreased expression of ScR-A mRNA and promoted cholesterol efflux, likely due to enhanced expression of ABCA1 and ABCG1 mRNAs. They then conducted further study of the mechanisms involved and found that the key miRNA mediating the upregulation of macrophage ABCA1 and ABCG1 by perivascular adipose tissue exosomes was miR-382-5p [
126].
Wang et al. showed that in mice with myocardial infarction induced by ligation of the left anterior descending coronary artery, intravenous administration of adipose-derived stem cell exosomes reduced infarct area, inhibited apoptosis and improved cardiac recovery
[2]. These myocardium-preserving results were found to be mediated via miR-205.
Evidence is beginning to accumulate that exosomes from different cell types found within obese adipose tissue affects whole body metabolism, and, indirectly, atherosclerotic risk [
127,
128,
129,
130]. For example, macrophages that reside within adipose tissue produce exosomes that transfer to adipocytes, and, in a murine model, lean mice treated with adipose tissue macrophage exosomes from obese mice became insulin resistant [
131]. Further evidence of the relationship among obesity, adipose tissue exosomes, and metabolism can be found in a human study showing that weight loss brought about after gastric bypass surgery changes the miRNA content of adipocyte-derived exosomes isolated from the peripheral blood [
132]. One year after surgery, 10 miRNAs that target insulin signaling pathways were found to be altered and this correlated with improvements in insulin sensitivity with better glucose homeostasis.
4. The Application of Exosomes in Treatment of Heart Disease
There is great interest in using the unique properties of exosomes to treat and prevent CVD
[3]. As discussed in our full paper, exosomes can affect key signaling pathways that are disrupted in the ischemic state and during myocardial infarction and they can change the proteome of the heart
[4] [5]. Exosomes may be used as vehicles for delivery of drugs, lipids, proteins, miRNAs and mRNAs to the heart and blood vessels. Advantages of exosomes in transporting these therapeutics include their biocompatibility, low immunogenicity and low toxicity as well as their ability to move through biological fluids
[6]. Further, they can be manipulated to target specific cell types by changing their surface markers
[7]. Initiatives to apply exosomes in human CVD treatment are slow to move forward, but several interventional studies have been initiated
[8].
Acknowledgments: We thank Ms. Lynn Drucker and Mr. Robert Buescher.