Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Hydrogen is widely considered as the fuel of the future. Due to the challenges present during hydrogen production using conventional processes and technologies, additional methods must be considered, like the use of microorganisms. One of the most promising technologies is dark fermentation, a process where microorganisms are utilized to produce hydrogen from biomass.
- biological hydrogen
- biowaste
- storage
1. Introduction
There is an increasing energy demand in the modern world. It is expected to be even more pronounced in the future as it is imposed by modern lifestyle and economic development [1]. However, natural resources for energy production are limited [2]. The major problem is posed by the use of non-renewable fossil fuel energy sources and the technologies used for production, which are direct polluters of the environment, leading to the generation of greenhouse gases and global warming [3][4][5].
The development of alternative, clean energy sources represents the right path toward solving the energy problems of humankind [6]. A practical alternative to fossil fuels is the utilization of hydrogen, which has been under discussion for a long time. It is included in the plans of countries in the European Union and beyond [7]. In line with the European Green Deal of 2020, the European Commission presented the Hydrogen Strategy for a Climate-Neutral Europe by 2050. Hydrogen is highlighted as one of the key levers for a successful energy transition and the European strategy for integrating energy systems [8]. The global hydrogen production in 2021 was about 90 megatons per year (Mt/y). However, most hydrogen is produced from fossil fuels, and less than 1% comes from renewable resources and microorganisms [9].
Hydrogen (H2) is the lightest element in the periodic table of elements and the most abundant chemical element in the universe [10]. It is a colorless, odorless, non-toxic, and highly flammable gas at standard pressure and density. It is difficult to find in its pure molecular form as it is lighter than air and rises from the atmosphere [11]. In the energy industry, hydrogen is nominated with a color designating the primary sources of energy used for hydrogen generation.
2. Hydrogen Production from Biomass
First-generation biomass mainly consists of Lignocellulosic biomass with high starch and sugar content, such as sweet sorghum, sugar beet, potato, wheat, pumpkin, and oilseed rape, as well as the residues and byproducts of their processing, which are traditionally utilized for food and feed [12]. Second-generation biomass is mainly represented by lignocellulosic biomass with low commercial value but high abundance. Third-generation biohydrogen production is derived from algal biomass rich in polysaccharides [13]. Algae have the ability to grow rapidly compared to other biomasses and sequester large amounts of CO2. In addition, they can contain a low amount of lignin and lignin oligomers [14][15], which makes them easy to hydrolyze [16]. The fourth-generation biomass feedstock comprises genetically modified organisms to improve biohydrogen production processes [17]. Second and third-generation biomasses, after genetic modification, become part of the fourth-generation feedstock. Genetic/metabolic modifications in microorganisms capable of contributing to the development of new technologies are achieved through genetic engineering or nanotechnology [18][19].
2.1. Lignocellulosic Biomass and Crop Residues Containing Sugars
Lignocellulosic biomass is considered “the most abundant organic component of the biosphere”, with an annual production of 1–5·1013 kg, which is considered an attractive and cheap substrate for the production of biofuels [20][21]. It is the most prevalent in nature and is present as hardwood, softwood, grasses, and agricultural residues. The estimated global annual yields of lignocellulosic biomass residues are more than 220 Bt [22], which is equivalent to 60–80 Bt of crude oil [23].
Based on dry matter composition, lignocellulosic biomass primarily consists of cellulose (40–60%), hemicellulose (20–40%), and lignin (10–25%) [24]. Cellulose and hemicellulose can be enzymatically hydrolyzed into smaller sugar molecules [25]. They mainly contain glucose and xylose, readily available for microorganisms that can effectively ferment glucose, and xylose are for bio-hydrogen production [26] (Figure 1).
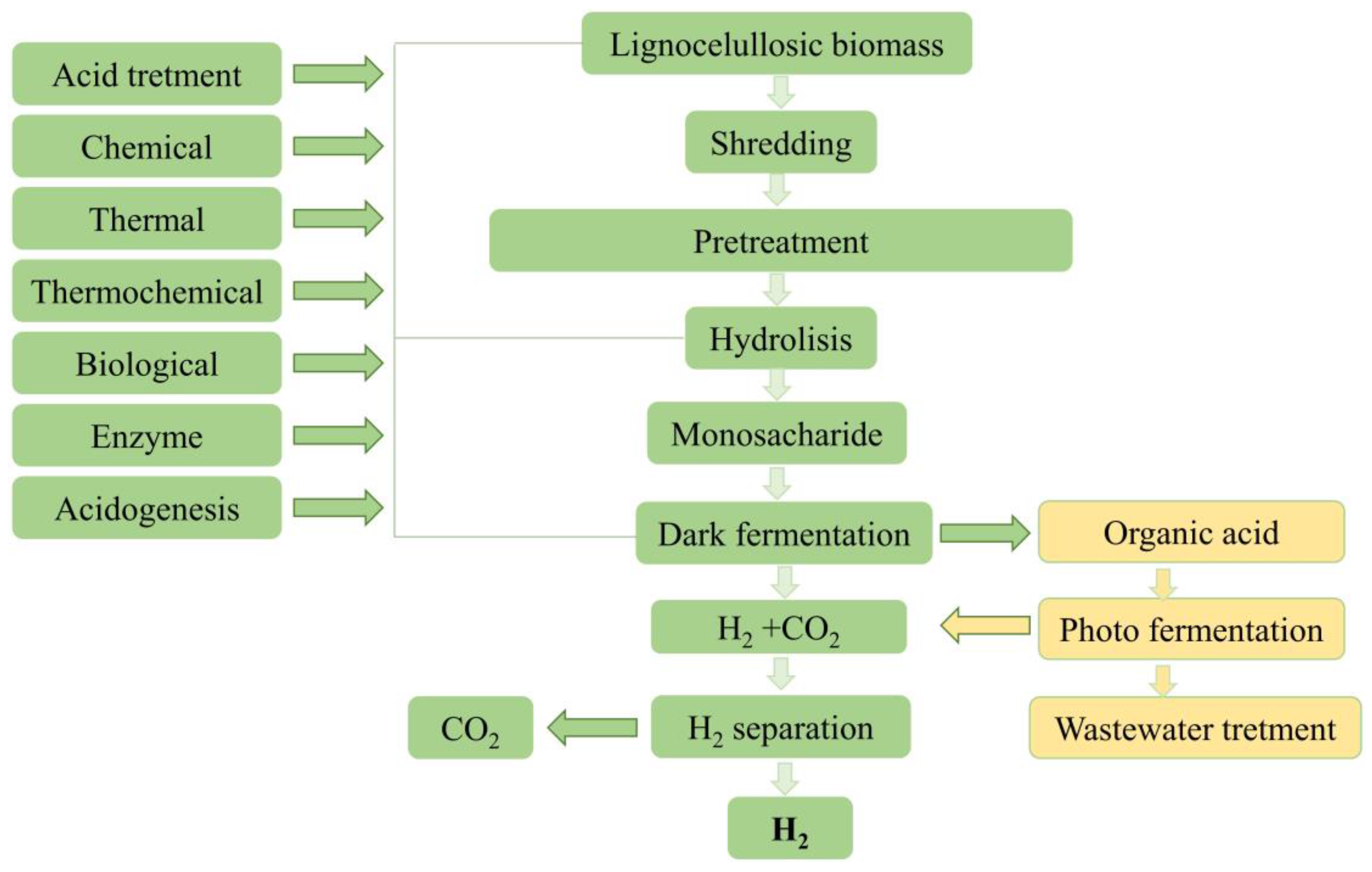
Figure 1. Process for obtaining hydrogen from lignocellulosic biomass.
In order to improve substrate hydrolysis and facilitate biological hydrogen production through dark fermentation in reactors, pretreatment of the lignocellulosic biomass matrix is used to release cellulose molecules into solution, breaking the crystal structure of cellulose and helping depolymerization [26]. Pretreatment can also be applied to the inoculum to enrich hydrogen-producing bacteria and inhibit hydrogen-consuming bacteria [27]. Mixed cultures have an advantage over pure cultures because they allow easier control of the process and higher substrate efficiency, but pretreatment should be performed in this case. Acid or alkaline treatment, thermal shock, aeration, chemical inhibition, or inhibition with long-chain fatty acids can be used to increase hydrogen production by dark fermentation. Pretreatment aims to suppress the growth of hydrogen consumers that do not form spores. Spore-forming hydrogen-producing bacteria survive this pretreatment [28].
Hydrogen can be produced from lignocellulosic biomass through dark fermentation with a yield close to the maximum theoretical yield of 4 moles of hydrogen per mole of hexose [23]. It is worth mentioning that a wide range of monosaccharides, disaccharides, and polysaccharides (including cellulose) can be used for hydrogen production [29].
Caldicellulosiruptor saccharolyticus is an example of an interesting bacterium for bio-hydrogen production. C. saccharolyticus is a thermophile, strictly anaerobic, Gram-positive, cellulolytic bacterium bio-hydrogen producer [29]. Genome analysis revealed that C. saccharolyticus has a high ability to hydrolyze polysaccharides such as cellulose, hemicellulose, pectin, and starch, along with a large number of ABC transporters for the uptake of monomeric and oligomeric sugars [30]. Catabolic pathways have been identified for a range of sugars, including rhamnose, fucose, arabinose, glucuronic acid, fructose, and galactose, which lead to the production of NADH and reduced ferredoxin. NADH and reduced ferredoxin later use two different hydrogenases to form hydrogen [31]. Whole-genome transcriptome analysis revealed significant regulation of the glycolytic pathway and ABC-type sugar transporters during growth on glucose and xylose, indicating that C. saccharolyticus coferments these sugars without repression based on glucose catabolite [20]. In addition, C. saccharolyticus is an extreme thermophile. It is known that H2 yields are higher in thermophilic than in mesophilic conditions, although the volumetric productivity is reversed [32]. Thus, transferring the metabolic pathway of C. saccharolyticus to a mesophilic bacterium would be advantageous. The role of genetic engineering in cellulolytic microorganisms, in fact, is significant and enables the redirection of metabolic pathways toward maximum hydrogen production [33]. For example, introducing mutations in mesophilic E. coli by inactivating the lactate dehydrogenase gene (ldhA) increases hydrogen production by 20–45% [34].
To increase H2 production in fermentation systems, modifications to metabolic engineering as the introduction/overexpression of genes (cellulases, hemicellulases, and lignases), can be used to increase the availability of carbohydrates for the cell, overexpression of enzymes that produce H2, and disruption of metabolic pathways that compete for reductive equivalents [23].
2.2. Wastewaters
Wastewater, particularly those from industrial facilities, presents a promising avenue for water treatment companies to apply methods of wastewater recycling to produce green hydrogen directly using microorganisms [35]. Wastewater consists of 70% organic compounds and 30% inorganic compounds, with organic compounds primarily being carbohydrates, fats, and proteins, which can be utilized through dark fermentation for hydrogen production [36]. In dark fermentation methods, bacteria such as Clostridium thermocellum break down organic materials and produce hydrogen [37].
The wastewater microbial consortium contains a large number of bacteria, some of which inhibit hydrogen production (i.e., hydrogenophilic methanogenesis) through consumption (homo-acetogens and methanogens) [35]. Therefore, for optimal hydrogen production, the activity of inhibitory microorganisms is suppressed or killed, most commonly through pre-heating of the inoculum [38] or, in a more cheap way, optimizing the parameters processes as the initial pH [39].
Potentially, wastewater constitutes readily accessible and inexpensive biomass from which hydrogen can be produced [40]. This would reduce the negative impact of wastewater treatment on the environment while increasing hydrogen production, thus making wastewater a fuel of the future [41]. Essentially, green hydrogen from wastewater has tremendous potential for further development [42].
Organic wastewater contains a potentially high energy content, with each kg of chemical oxygen demand producing about 1.4 × 107 kg of metabolic heat, which has immense practical significance [43][44][45]. It has been noted that the energy contained in wastewater is 9.3 times greater than the energy consumed for its treatment [46]. If 10% of the energy can be utilized, it could power the wastewater treatment facility. As a result, energy extraction from organic waste is undoubtedly critical for developing low-carbon “energy saving and emissions reduction” models and for developing renewable energy [43].
3. Hydrogen Management
It would be ideal to use bio-hydrogen on the production site [47]. However, these are mainly energy generation systems by combustion, and the produced energy is transferred to other systems through a heat exchanger: biological, technical, and physical processes [48]. In this way, energy losses are minimized, and the costs of preparation for transport, transport, and storage, as well as the costs of re-releasing pure hydrogen as a fuel at the destination, are avoided [49].
However, quantities of produced gas from 100,000 m3 to several million cubic meters must be stored in natural or technically advanced geological formations, ensuring the conditions of impermeability, preservation of the purity of stored hydrogen from bacterial, organic, and inorganic pollution, and the possibility of increasing storage space [50].
3.1. Hydrogen Storage
The final choice of the method and form of hydrogen handling—storage depends on its final use, the form of energy, and the energy conversion method [51].
3.1.1. Natural Caves and Salt Mine Caves
The walls of such caves ensure the impermeability and preservation of the storage gas’s purity [52]. Such storage facilities are already used in the USA and the UK [53]. Such a form of storage is considered the most economical option and the safest gas storage facility that is well protected from external influences (terrorist attacks, fires, and military actions) [53]. The capacities of such caves are around one million cubic meters [54]. In general, pure hydrogen is in the form of fuel cells ready for technological application (vehicles, etc.) [55].
3.1.2. Underground Hydrogen Storage in a Gas Mixture
Besides its nascent form, hydrogen can be stored in depleted natural gas deposits (natural gas: a mixture of methane and higher homologs) or a mixture with methane, CO2, and CO (synthetic gas) [56], as well as in a mixture with city gas (methane, CO) [57].
All of these forms of stored gas can be utilized in gas turbines or fuel cells to produce electrical energy [58]. Such mixtures can be stored in salt caverns and aquifers (so-called depleted geological environments saturated with free subterranean waters) [59]. They form in water-impermeable rocks as well as in natural gas storage facilities (hydrogen content of 5–15%) [51].
Hydrogen must be separated from the mixture with natural gas in pure form for further use [60]. This can be achieved through membrane filtration, adsorption, and electrochemical separation [61].
3.1.3. Special Cases of Hydrogen Storage in Subsurface Reactors for the Methanation Process
In underground storage sites of natural gas and aquifers, hydrogen and carbon dioxide are subjected to hydrogenotrophic methanogenic bacteria that, through the process of methanization, create methane [62]. This process takes place at low temperatures, which is more acceptable than some high-temperature processes with catalysts [63].
3.2. Hydrogen Transport
Hydrogen transport can be considered a special form of hydrogen storage [64]. Long-distance transportation is carried out based on the technical principles of natural gas transport [65]. Hydrogen can be transported as a liquefied gas or as hydrogen converted into ammonia, methanol, or another transportable fluid. However, additional costs such as “energy loss” are incurred [66].
The most favorable cost-to-output ratio for hydrogen transport is transporting in liquefied form or the form of ammonia, with the possibility of hydrogen production via cracking at the consumption site [67]. However, the most significant drawbacks of these technologies are the costs of liquefaction and cracking, and the more promising technology is liquefaction [68]. Currently, efforts are underway to develop technologies that reduce costs when hydrogen is used as an energy source rather than a raw material in the form of ammonia [69].
Hydrogen can also be transported and stored using other organic carriers such as methanol and methylcyclohexane [70]. In hydrogen transport systems, such as pipelines, the phenomenon of diffusion occurs in the material the pipeline is made of [71]. This phenomenon is known as “hydrogen brittleness” and manifests as a reduction in the mechanical properties of pipelines. The degree of hydrogen brittleness depends on the operation regime of the pipeline (fluctuation of pressures, the type of material the pipeline is made of) [72]. Therefore, processes for handling hydrogen after production in terms of transport and storage represent an enormous challenge in finding the most efficient, safe, and cost-effective methods [73]. However, given the growing commitment to hydrogen as an energy source of the future, researchers are confident that optimal solutions will also be found for handling hydrogen [47].
3.3. Hydrogen Economics
According to Kayfeci et al., hydrogen production can cost between 1.25 USD/kg and 23.27 USD/kg, respectively [69][74]. The choice of the production method, in this case, is impacting the H2 production costs, where fossil fuel-based technologies tend to have relatively low costs, 1.34–2.27 USD/kg, while solar-based technologies (PV, thermal, solar thermolysis, and photo-thermolysis) tend to be with the highest cost varying between 5.78–23.27 USD/kg) [75]. Among the most competitive options are indirect bio-photolysis, with a cost of 1.42 USD/kg, and direct bio-photolysis, with a cost of 2.13 USD/kg [76]. The dark fermentation method is estimated to cost 2.57 USD/kg, which is in the same range as nuclear thermolysis option 2.17–2.63 USD/kg, while nuclear electrolysis is estimated to have costs 4.15–7.00 USD/kg, respectively [77] (Table 1).
| Process/Method | Cost (USD/kg) |
|---|---|
| Dark fermentation | 2.57 |
| Photo-fermentation | 2.83 |
| Biomass pyrolysis | 1.25–2.20 |
| Biomass gasification | 1.77–2.05 |
| Direct biophotolysis | 2.13 |
| Indirect biophotolysis | 1.42 |
| Dark fermentation | 2.57 |
| Photo-fermentation | 2.83 |
This entry is adapted from the peer-reviewed paper 10.3390/en16083321
References
- Ghasemian, S.; Faridzad, A.; Abbaszadeh, P.; Taklif, A.; Ghasemi, A.; Hafezi, R. An overview of global energy scenarios by 2040: Identifying the driving forces using cross-impact analysis method. Int. J. Environ. Sci. Technol. 2020, 1–24.
- Ahmadov, A.K.; van der Borg, C. Do natural resources impede renewable energy production in the EU? A mixed-methods analysis. Energy Policy 2019, 126, 361–369.
- Perera, F. Pollution from Fossil-Fuel Combustion is the Leading Environmental Threat to Global Pediatric Health and Equity: Solutions Exist. Int. J. Environ. Res. Public Health 2018, 15, 16.
- Kumar, M.; Kumar, M. Social, Economic, and Environmental Impacts of Renewable Energy Resources. In Wind Solar Hybrid Renewable Energy System; Okedu, K.E., Tahour, A., Aissaou, A.G., Eds.; IntechOpen: London, UK, 2020; ISBN 978-1-78984-591-4.
- Lamb, W.F.; Wiedmann, T.; Pongratz, J.; Andrew, R.; Crippa, M.; Olivier, J.G.J.; Wiedenhofer, D.; Mattioli, G.; Al Khourdajie, A.; House, J.; et al. A review of trends and drivers of greenhouse gas emissions by sector from 1990 to 2018. Environ. Res. Lett. 2021, 16, 073005.
- Seetharaman; Moorthy, K.; Patwa, N.; Saravanan; Gupta, Y. Breaking barriers in deployment of renewable energy. Heliyon 2019, 5, e01166.
- Gielen, D.; Boshell, F.; Saygin, D.; Bazilian, M.D.; Wagner, N.; Gorini, R. The role of renewable energy in the global energy transformation. Energy Strateg. Rev. 2019, 24, 38–50.
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. A Hydrogen Strategy for a Climate EN Neutral Europe; European Commission: Brussels, Belgium, 2020.
- IRENA—International Renewable Energy Agency Hydrogen. Available online: https://www.irena.org/Energy-Transition/Technology/Hydrogen (accessed on 8 February 2023).
- Chen, R.; Wang, Y.Z.; Liao, Q.; Zhu, X.; Xu, T.F. Hydrolysates of lignocellulosic materials for biohydrogen production. BMB Rep. 2013, 46, 244–251.
- Panić, I.; Cuculić, A.; Ćelić, J. Color-Coded Hydrogen: Production and Storage in Maritime Sector. J. Mar. Sci. Eng. 2022, 10, 1995.
- Saratale, G.D.; Saratale, R.G.; Banu, J.R.; Chang, J.-S. Biohydrogen Production From Renewable Biomass Resources. In Biomass, Biofuels, Biochemicals, Biohydrogen; Pandey, A., Mohan, S.V., Chang, J.-S., Hallenbeck, P.C., Larroche, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 247–277.
- Ortigueira, J.; Alves, L.; Gouveia, L.; Moura, P. Third generation biohydrogen production by Clostridium butyricum and adapted mixed cultures from Scenedesmus obliquus microalga biomass. Fuel 2015, 153, 128–134.
- Kabir, F.; Gulfraz, M.; Raja, G.; Inam-ul-Haq, M.; Batool, I.; Awais, M.; Habiba, U.; Gul, H. Comparative study on the usability of lignocellulosic and algal biomass for production of alcoholic fuels. BioResources 2019, 14, 8135–8154.
- Allouache, A.; Majda, A.; Toudert, A.Z.; Amrane, A.; Ballesteros, M. Cellulosic bioethanol production from Ulva lactuca macroalgae. Cellul. Chem. Technol. 2021, 55, 629–635.
- Sarwer, A.; Hamed, S.M.; Osman, A.I.; Jamil, F.; Al-Muhtaseb, A.H.; Alhajeri, N.S.; Rooney, D.W. Algal biomass valorization for biofuel production and carbon sequestration: A review. Environ. Chem. Lett. 2022, 20, 2797–2851.
- Shokravi, H.; Shokravi, Z.; Heidarrezaei, M.; Ong, H.C.; Rahimian Koloor, S.S.; Petrů, M.; Lau, W.J.; Ismail, A.F. Fourth generation biofuel from genetically modified algal biomass: Challenges and future directions. Chemosphere 2021, 285, 131535.
- Ahmed, S.F.; Rafa, N.; Mofijur, M.; Badruddin, I.A.; Inayat, A.; Ali, M.S.; Farrok, O.; Yunus Khan, T.M. Biohydrogen Production From Biomass Sources: Metabolic Pathways and Economic Analysis. Front. Energy Res. 2021, 9, 529.
- Govindasamy, R.; Gayathiri, E.; Sankar, S.; Venkidasamy, B.; Prakash, P.; Rekha, K.; Savaner, V.; Pari, A.; Thirumalaivasan, N.; Thiruvengadam, M. Emerging Trends of Nanotechnology and Genetic Engineering in Cyanobacteria to Optimize Production for Future Applications. Life 2022, 12, 2013.
- Björkmalm, J.; Byrne, E.; Van Niel, E.W.J.; Willquist, K. A non-linear model of hydrogen production by Caldicellulosiruptor saccharolyticus for diauxic-like consumption of lignocellulosic sugar mixtures. Biotechnol. Biofuels 2018, 11, 175.
- Lin, K.; Luque, R.; Dutta, N.; Usman, M.; Luo, G.; Zhang, S. An Insight into Valorization of Lignocellulosic Biomass by Optimization with the Combination of Hydrothermal (HT) and Biological Techniques: A Review. Sustain. Chem. 2022, 3, 35–55.
- Kumar, G.; Bakonyi, P.; Periyasamy, S.; Kim, S.H.; Nemestóthy, N.; Bélafi-Bakó, K. Lignocellulose biohydrogen: Practical challenges and recent progress. Renew. Sustain. Energy Rev. 2015, 44, 728–737.
- Singh, A.; Sevda, S.; Abu Reesh, I.M.; Vanbroekhoven, K.; Rathore, D.; Pant, D. Biohydrogen Production from Lignocellulosic Biomass: Technology and Sustainability. Energies 2015, 8, 13062–13080.
- Ning, P.; Yang, G.; Hu, L.; Sun, J.; Shi, L.; Zhou, Y.; Wang, Z.; Yang, J. Recent advances in the valorization of plant biomass. Biotechnol. Biofuels 2021, 14, 102.
- Su, T.; Zhao, D.; Khodadadi, M.; Len, C. Lignocellulosic biomass for bioethanol: Recent advances, technology trends, and barriers to industrial development. Curr. Opin. Green Sustain. Chem. 2020, 24, 56–60.
- Honarmandrad, Z.; Kucharska, K.; Gębicki, J. Processing of Biomass Prior to Hydrogen Fermentation and Post-Fermentative Broth Management. Molecules 2022, 27, 7658.
- Dauptain, K.; Trably, E.; Santa-Catalina, G.; Bernet, N.; Carrere, H. Role of indigenous bacteria in dark fermentation of organic substrates. Bioresour. Technol. 2020, 313, 123665.
- Rafieenia, R.; Lavagnolo, M.C.; Pivato, A. Pre-treatment technologies for dark fermentative hydrogen production: Current advances and future directions. Waste Manag. 2018, 71, 734–748.
- Bielen, A.A.M.; Verhaart, M.R.A.; van der Oost, J.; Kengen, S.W.M. Biohydrogen Production by the Thermophilic Bacterium Caldicellulosiruptor saccharolyticus: Current Status and Perspectives. Life 2013, 3, 52–85.
- Pecorini, I.; Baldi, F.; Iannelli, R. Biochemical Hydrogen Potential Tests Using Different Inocula. Sustainability 2019, 11, 622.
- Van De Werken, H.J.G.; Verhaart, M.R.A.; VanFossen, A.L.; Willquist, K.; Lewis, D.L.; Nichols, J.D.; Goorissen, H.P.; Mongodin, E.F.; Nelson, K.E.; Van Niel, E.W.J.; et al. Hydrogenomics of the extremely thermophilic bacterium Caldicellulosiruptor saccharolyticus. Appl. Environ. Microbiol. 2008, 74, 6720–6729.
- Vasmara, C.; Galletti, S.; Cianchetta, S.; Ceotto, E. Advancements in Giant Reed (Arundo donax L.) Biomass Pre-Treatments for Biogas Production: A Review. Energies 2023, 16, 949.
- Dadwal, A.; Sharma, S.; Satyanarayana, T. Progress in Ameliorating Beneficial Characteristics of Microbial Cellulases by Genetic Engineering Approaches for Cellulose Saccharification. Front. Microbiol. 2020, 11, 1387.
- Hallenbeck, P.C.; Ghosh, D. Improvements in fermentative biological hydrogen production through metabolic engineering. J. Environ. Manag. 2012, 95, S360–S364.
- Bhatia, R.K.; Sakhuja, D.; Mundhe, S.; Walia, A. Renewable Energy Products through Bioremediation of Wastewater. Sustainability 2020, 12, 7501.
- Preethi; Usman, T.M.M.; Rajesh Banu, J.; Gunasekaran, M.; Kumar, G. Biohydrogen production from industrial wastewater: An overview. Bioresour. Technol. Rep. 2019, 7, 100287.
- Łukajtis, R.; Hołowacz, I.; Kucharska, K.; Glinka, M.; Rybarczyk, P.; Przyjazny, A.; Kamiński, M. Hydrogen production from biomass using dark fermentation. Renew. Sustain. Energy Rev. 2018, 91, 665–694.
- Singh, H.; Tomar, S.; Qureshi, K.A.; Jaremko, M.; Rai, P.K. Recent Advances in Biomass Pretreatment Technologies for Biohydrogen Production. Energies 2022, 15, 999.
- Vasmara, C.; Marchetti, R. Initial pH influences in-batch hydrogen production from scotta permeate. Int. J. Hydrogen Energy 2017, 42, 14400–14408.
- Qyyum, M.A.; Ismail, S.; Ni, S.Q.; Ihsanullah, I.; Ahmad, R.; Khan, A.; Tawfik, A.; Nizami, A.S.; Lee, M. Harvesting biohydrogen from industrial wastewater: Production potential, pilot-scale bioreactors, commercialization status, techno-economics, and policy analysis. J. Clean. Prod. 2022, 340, 130809.
- Akbarzadeh, R.; Adeniran, J.A.; Lototskyy, M.; Asadi, A. Simultaneous brewery wastewater treatment and hydrogen generation via hydrolysis using Mg waste scraps. J. Clean. Prod. 2020, 276, 123198.
- Pitchaimuthu, S.; Sridharan, K.; Nagarajan, S.; Ananthraj, S.; Robertson, P.; Kuehnel, M.F.; Irabien, Á.; Maroto-Valer, M. Solar Hydrogen Fuel Generation from Wastewater—Beyond Photoelectrochemical Water Splitting: A Perspective. Energies 2022, 15, 7399.
- Qu, X.; Zeng, H.; Gao, Y.; Mo, T.; Li, Y. Bio-hydrogen production by dark anaerobic fermentation of organic wastewater. Front. Chem. 2022, 10, 1095.
- Zhang, J.; Kan, X.; Shen, Y.; Loh, K.C.; Wang, C.H.; Dai, Y.; Tong, Y.W. A hybrid biological and thermal waste-to-energy system with heat energy recovery and utilization for solid organic waste treatment. Energy 2018, 152, 214–222.
- Kumar, A.; Samadder, S.R. Performance evaluation of anaerobic digestion technology for energy recovery from organic fraction of municipal solid waste: A review. Energy 2020, 197, 117253.
- Pires, M.; Meloni, E.; Skov, I.R.; Vilardi, G.; Zuorro, A.; Belikov, J.; Perea-Moreno, A.-J.; Toczyłowska-Mamí Nska, R.; Mamí Nski, M.Ł. Wastewater as a Renewable Energy Source—Utilisation of Microbial Fuel Cell Technology. Energies 2022, 15, 6928.
- Qazi, U.Y. Future of Hydrogen as an Alternative Fuel for Next-Generation Industrial Applications; Challenges and Expected Opportunities. Energies 2022, 15, 4741.
- Kanwal, F.; Torriero, A.A.J. Biohydrogen-A Green Fuel for Sustainable Energy Solutions. Energies 2022, 15, 7783.
- Vidas, L.; Castro, R. Recent Developments on Hydrogen Production Technologies: State-of-the-Art Review with a Focus on Green-Electrolysis. Appl. Sci. 2021, 11, 11363.
- Wollin, K.M.; Damm, G.; Foth, H.; Freyberger, A.; Gebel, T.; Mangerich, A.; Gundert-Remy, U.; Partosch, F.; Röhl, C.; Schupp, T.; et al. Critical evaluation of human health risks due to hydraulic fracturing in natural gas and petroleum production. Arch. Toxicol. 2020, 94, 967–1016.
- Muhammed, N.S.; Haq, B.; Al Shehri, D.; Al-Ahmed, A.; Rahman, M.M.; Zaman, E. A review on underground hydrogen storage: Insight into geological sites, influencing factors and future outlook. Energy Rep. 2022, 8, 461–499.
- Li, J.; Shi, X.; Yang, C.; Li, Y.; Wang, T.; Ma, H.; Shi, H.; Li, J.; Liu, J. Repair of irregularly shaped salt cavern gas storage by re-leaching under gas blanket. J. Nat. Gas Sci. Eng. 2017, 45, 848–859.
- Małachowska, A.; Łukasik, N.; Mioduska, J.; Gębicki, J. Hydrogen Storage in Geological Formations—The Potential of Salt Caverns. Energies 2022, 15, 5038.
- Sampim, T.; Kokkaew, N.; Parnphumeesup, P. Risk Management in Biomass Power Plants Using Fuel Switching Flexibility. Energy Procedia 2017, 138, 1099–1104.
- Yue, M.; Lambert, H.; Pahon, E.; Roche, R.; Jemei, S.; Hissel, D. Hydrogen energy systems: A critical review of technologies, applications, trends and challenges. Renew. Sustain. Energy Rev. 2021, 146, 111180.
- Biswas, S.; Kulkarni, A.P.; Giddey, S.; Bhattacharya, S. A Review on Synthesis of Methane as a Pathway for Renewable Energy Storage With a Focus on Solid Oxide Electrolytic Cell-Based Processes. Front. Energy Res. 2020, 8, 229.
- Mahajan, D.; Tan, K.; Venkatesh, T.; Kileti, P.; Clayton, C.R. Hydrogen Blending in Gas Pipeline Networks—A Review. Energies 2022, 15, 3582.
- Dominguez-Gonzalez, G.; Muñoz-Hernandez, J.I.; Bunn, D.; Garcia-Checa, C.J. Integration of Hydrogen and Synthetic Natural Gas within Legacy Power Generation Facilities. Energies 2022, 15, 4485.
- Molíková, A.; Vítězová, M.; Vítěz, T.; Buriánková, I.; Huber, H.; Dengler, L.; Hanišáková, N.; Onderka, V.; Urbanová, I. Underground gas storage as a promising natural methane bioreactor and reservoir? J. Energy Storage 2022, 47, 103631.
- Balsalobre-Lorente, D.; Radulescu, M.; Shahzad, U.; Rehman, A.; Neacsa, A.; Eparu, C.N.; Stoica, D.B. Hydrogen—Natural Gas Blending in Distribution Systems—An Energy, Economic, and Environmental Assessment. Energies 2022, 15, 6143.
- Vermaak, L.; Neomagus, H.W.J.P.; Bessarabov, D.G. Hydrogen Separation and Purification from Various Gas Mixtures by Means of Electrochemical Membrane Technology in the Temperature Range 100–160 °C. Membranes 2021, 11, 282.
- Strobel, G.; Hagemann, B.; Huppertz, T.M.; Ganzer, L. Underground bio-methanation: Concept and potential. Renew. Sustain. Energy Rev. 2020, 123, 109747.
- Elberry, A.M.; Thakur, J.; Santasalo-Aarnio, A.; Larmi, M. Large-scale compressed hydrogen storage as part of renewable electricity storage systems. Int. J. Hydrogen Energy 2021, 46, 15671–15690.
- Ustolin, F.; Campari, A.; Taccani, R. An Extensive Review of Liquid Hydrogen in Transportation with Focus on the Maritime Sector. J. Mar. Sci. Eng. 2022, 10, 1222.
- Agyekum, E.B.; Nutakor, C.; Agwa, A.M.; Kamel, S. A Critical Review of Renewable Hydrogen Production Methods: Factors Affecting Their Scale-Up and Its Role in Future Energy Generation. Membranes 2022, 12, 173.
- Aziz, M. Liquid Hydrogen: A Review on Liquefaction, Storage, Transportation, and Safety. Energies 2021, 14, 5917.
- Aziz, M.; TriWijayanta, A.; Nandiyanto, A.B.D. Ammonia as Effective Hydrogen Storage: A Review on Production, Storage and Utilization. Energies 2020, 13, 3062.
- Van Hoecke, L.; Laffineur, L.; Campe, R.; Perreault, P.; Verbruggen, S.W.; Lenaerts, S. Challenges in the use of hydrogen for maritime applications. Energy Environ. Sci. 2021, 14, 815–843.
- Rizi, H.A.Y.; Shin, D.; Hossein Ali, Y.R.; Shin, D. Green Hydrogen Production Technologies from Ammonia Cracking. Energies 2022, 15, 8246.
- Abdin, Z.; Tang, C.; Liu, Y.; Catchpole, K. Large-scale stationary hydrogen storage via liquid organic hydrogen carriers. iScience 2021, 24, 102966.
- Laureys, A.; Depraetere, R.; Cauwels, M.; Depover, T.; Hertelé, S.; Verbeken, K. Use of existing steel pipeline infrastructure for gaseous hydrogen storage and transport: A review of factors affecting hydrogen induced degradation. J. Nat. Gas Sci. Eng. 2022, 101, 104534.
- Bethoux, O. Hydrogen Fuel Cell Road Vehicles and Their Infrastructure: An Option towards an Environmentally Friendly Energy Transition. Energies 2020, 13, 6132.
- Tashie-Lewis, B.C.; Nnabuife, S.G. Hydrogen Production, Distribution, Storage and Power Conversion in a Hydrogen Economy—A Technology Review. Chem. Eng. J. Adv. 2021, 8, 100172.
- Kayfeci, M.; Keçebaş, A.; Bayat, M. Hydrogen production. In Solar Hydrogen Production: Processes, Systems and Technologies; Calise, F., D’Accadia, M.D., Santarelli, M., Lanzini, A., Ferrero, D., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 45–83.
- Vostakola, F.; Salamatinia, M.; Horri, A.; Fallah Vostakola, M.; Salamatinia, B.; Horri, B.A. A Review on Recent Progress in the Integrated Green Hydrogen Production Processes. Energies 2022, 15, 1209.
- Talapko, J.; Talapko, D.; Matić, A.; Škrlec, I. Microorganisms as New Sources of Energy. Energies 2022, 15, 6365.
- Karthikeyan, B.; Gokuladoss, V. Fusion of Vermicompost and Sewage Sludge as Dark Fermentative Biocatalyst for Biohydrogen Production: A Kinetic Study. Energies 2022, 15, 6917.
This entry is offline, you can click here to edit this entry!
