The blood-brain barrier (BBB) is one of the most selective endothelial barriers that protect the brain and maintains homeostasis in neural microenvironments. This barrier restricts the passage of molecules into the brain, except for gaseous or extremely small hydrophobic molecules. Thus, the BBB hinders the delivery of drugs with large molecular weights for the treatment of brain cancers. Various methods have been used to deliver drugs to the brain by circumventing the BBB; however, they have limitations such as drug diversity and low delivery efficiency. To overcome this challenge, microbubbles (MBs)-based drug delivery systems have garnered a lot of interest in recent years. MBs are widely used as contrast agents and are recently being researched as a vehicle for delivering drugs, proteins, and gene complexes. The MBs are 1–10 μm in size and consist of a gas core and an organic shell, which cause physical changes, such as bubble expansion, contraction, vibration, and collapse, in response to ultrasound.
- microbubble
- ultrasound
- brain cancer
- blood-brain barrier
- drug delivery
1. Introduction
2. Structure and Composition of MBs
2.1. Core Structure
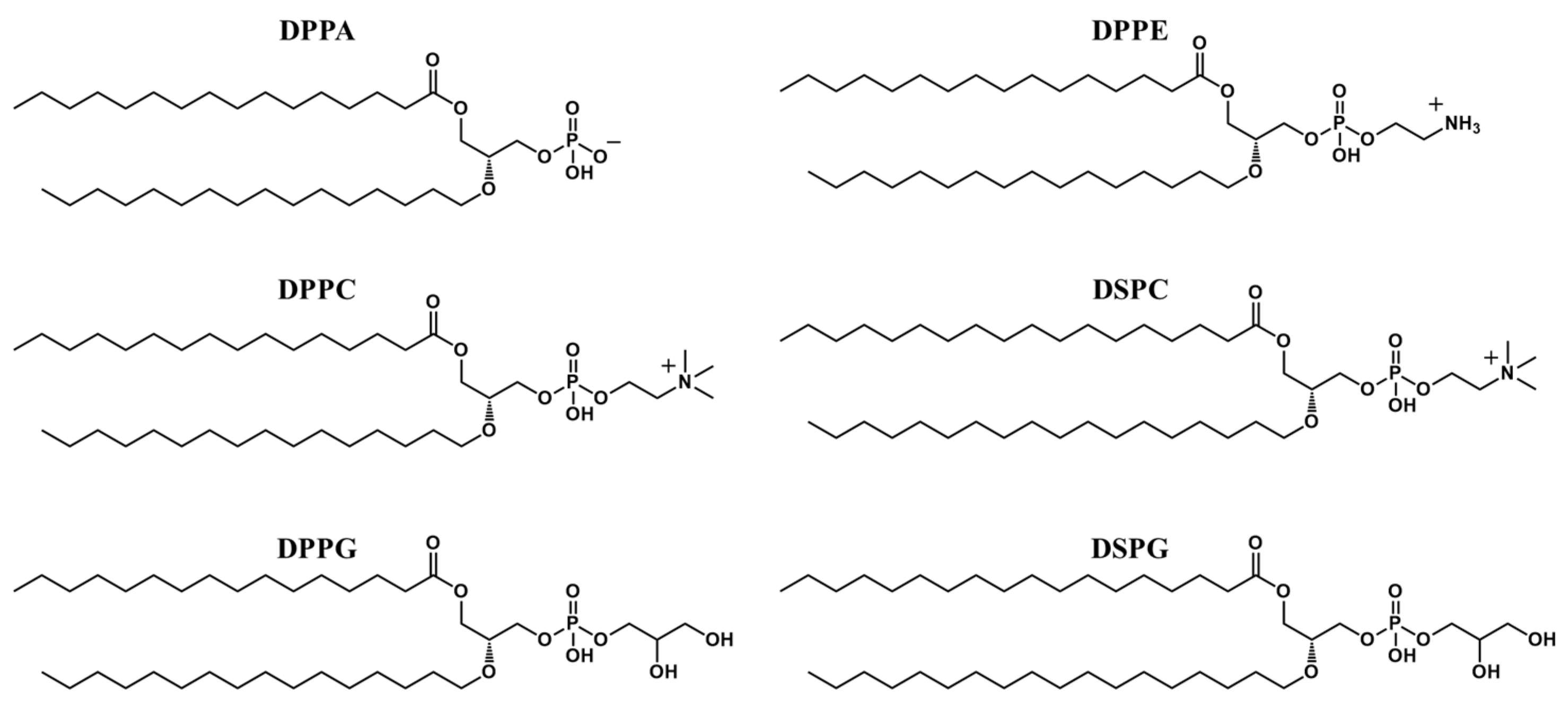
2.2. Shell Structure
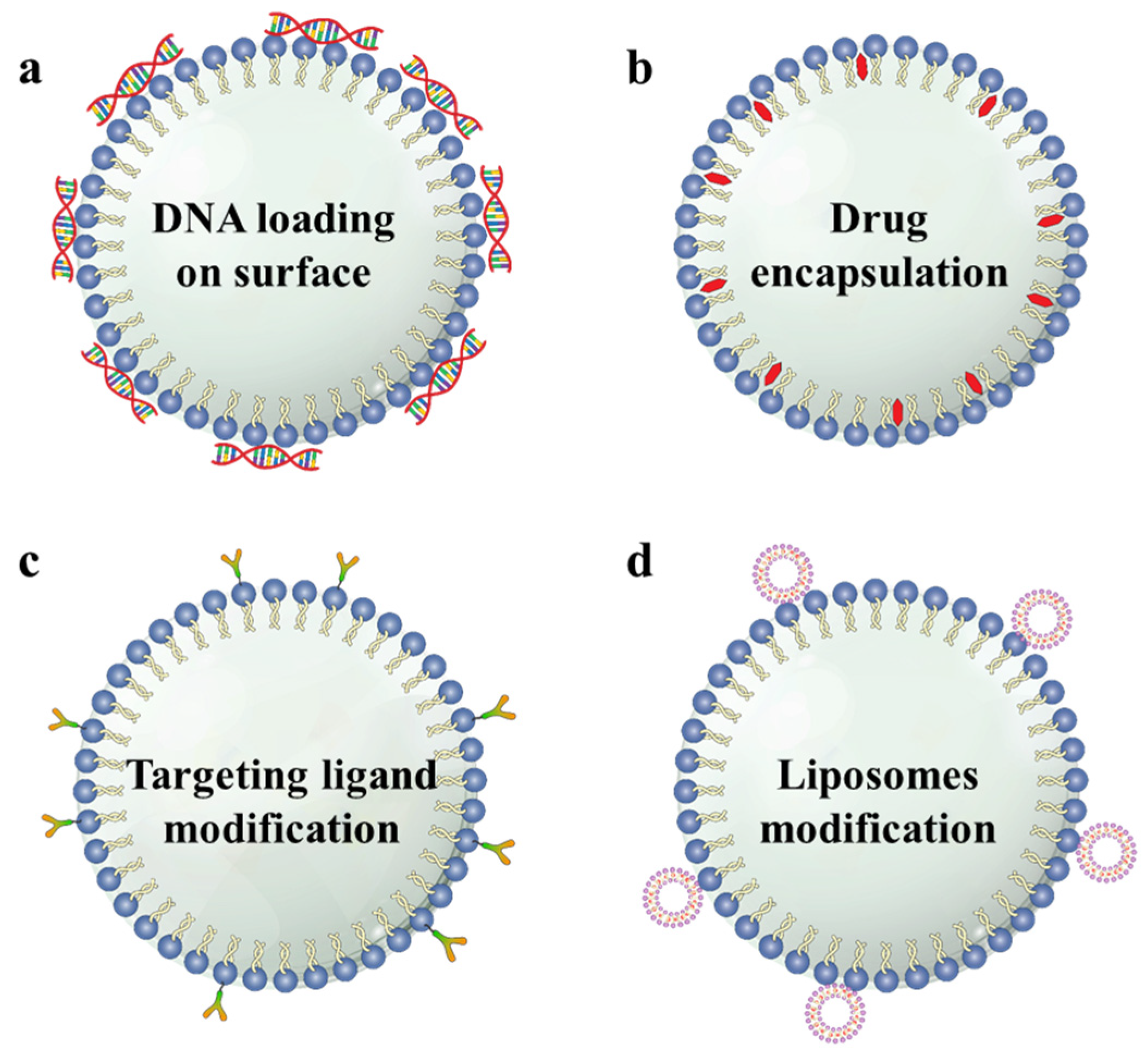
2.3. Multi-Functionalization of MBs
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics15020698
References
- Lockman, P.; Mumper, R.; Khan, M.; Allen, D. Nanoparticle technology for drug delivery across the blood-brain barrier. Drug Dev. Ind. Pharm. 2002, 28, 1–13.
- Banks, W.A. From blood-brain barrier to blood-brain interface: New opportunities for CNS drug delivery. Nat. Rev. Drug Discov. 2016, 15, 275–292.
- Parodi, A.; Rudzinska, M.; Deviatkin, A.A.; Soond, S.M.; Baldin, A.V.; Zamyatnin, A.A., Jr. Established and Emerging Strategies for Drug Delivery Across the Blood-Brain Barrier in Brain Cancer. Pharmaceutics 2019, 11, 245.
- Steeg, P.S. The blood-tumour barrier in cancer biology and therapy. Nat. Rev. Clin. Oncol. 2021, 18, 696–714.
- Pandit, R.; Chen, L.; Gotz, J. The blood-brain barrier: Physiology and strategies for drug delivery. Adv. Drug Deliv. Rev. 2020, 165–166, 1–14.
- Abbott, N.J. Blood-brain barrier structure and function and the challenges for CNS drug delivery. J. Inherit. Metab. Dis. 2013, 36, 437–449.
- McCrorie, P.; Vasey, C.E.; Smith, S.J.; Marlow, M.; Alexander, C.; Rahman, R. Biomedical engineering approaches to enhance therapeutic delivery for malignant glioma. J. Control. Release 2020, 328, 917–931.
- Schoen Jr, S.; Kilinc, M.S.; Lee, H.; Guo, Y.; Degertekin, F.L.; Woodworth, G.F.; Arvanitis, C. Towards controlled drug delivery in brain tumors with microbubble-enhanced focused ultrasound. Adv. Drug Delivery Rev. 2022, 180, 114043.
- Alonso, A.; Reinz, E.; Jenne, J.W.; Fatar, M.; Schmidt-Glenewinkel, H.; Hennerici, M.G.; Meairs, S. Reorganization of gap junctions after focused ultrasound blood-brain barrier opening in the rat brain. J. Cereb. Blood Flow Metab. 2010, 30, 1394–1402.
- Mitusova, K.; Peltek, O.O.; Karpov, T.E.; Muslimov, A.R.; Zyuzin, M.V.; Timin, A.S. Overcoming the blood-brain barrier for the therapy of malignant brain tumor: Current status and prospects of drug delivery approaches. J. Nanobiotechnol. 2022, 20, 412.
- Agrahari, V.; Agrahari, V.; Mitra, A.K. Nanocarrier fabrication and macromolecule drug delivery: Challenges and opportunities. Ther. Deliv. 2016, 7, 257–278.
- Wu, S.K.; Tsai, C.L.; Huang, Y.; Hynynen, K. Focused Ultrasound and Microbubbles-Mediated Drug Delivery to Brain Tumor. Pharmaceutics 2020, 13, 15.
- Sheikov, N.; McDannold, N.; Sharma, S.; Hynynen, K. Effect of focused ultrasound applied with an ultrasound contrast agent on the tight junctional integrity of the brain microvascular endothelium. Ultrasound Med. Biol. 2008, 34, 1093–1104.
- Liu, H.L.; Fan, C.H.; Ting, C.Y.; Yeh, C.K. Combining microbubbles and ultrasound for drug delivery to brain tumors: Current progress and overview. Theranostics 2014, 4, 432–444.
- Upton, D.H.; Ung, C.; George, S.M.; Tsoli, M.; Kavallaris, M.; Ziegler, D.S. Challenges and opportunities to penetrate the blood-brain barrier for brain cancer therapy. Theranostics 2022, 12, 4734–4752.
- Bai, M.; Dong, Y.; Huang, H.; Fu, H.; Duan, Y.; Wang, Q.; Du, L. Tumour targeted contrast enhanced ultrasound imaging dual-modal microbubbles for diagnosis and treatment of triple negative breast cancer. RSC Adv. 2019, 9, 5682–5691.
- Song, K.H.; Harvey, B.K.; Borden, M.A. State-of-the-art of microbubble-assisted blood-brain barrier disruption. Theranostics 2018, 8, 4393–4408.
- Dasgupta, A.; Liu, M.; Ojha, T.; Storm, G.; Kiessling, F.; Lammers, T. Ultrasound-mediated drug delivery to the brain: Principles, progress and prospects. Drug Discov. Today Technol. 2016, 20, 41–48.
- Stockwell, J.; Abdi, N.; Lu, X.; Maheshwari, O.; Taghibiglou, C. Novel central nervous system drug delivery systems. Chem. Biol. Drug Des. 2014, 83, 507–520.
- Yi, S.; Han, G.; Shang, Y.; Liu, C.; Cui, D.; Yu, S.; Liao, B.; Ao, X.; Li, G.; Li, L. Microbubble-mediated ultrasound promotes accumulation of bone marrow mesenchymal stem cell to the prostate for treating chronic bacterial prostatitis in rats. Sci. Rep. 2016, 6, 19745.
- Isik, U.; Aydogan Avsar, P.; Aktepe, E.; Doguc, D.K.; Kilic, F.; Buyukbayram, H.I. Serum zonulin and claudin-5 levels in children with obsessive-compulsive disorder. Nord. J. Psychiatry 2020, 74, 346–351.
- Lotfi, S.; Patel, A.S.; Mattock, K.; Egginton, S.; Smith, A.; Modarai, B. Towards a more relevant hind limb model of muscle ischaemia. Atherosclerosis 2013, 227, 1–8.
- Wang, F.; Wei, X.X.; Chang, L.S.; Dong, L.; Wang, Y.L.; Li, N.N. Ultrasound Combined With Microbubbles Loading BDNF Retrovirus to Open BloodBrain Barrier for Treatment of Alzheimer’s Disease. Front. Pharmacol. 2021, 12, 615104.
- Chien, C.Y.; Xu, L.; Pacia, C.P.; Yue, Y.; Chen, H. Blood-brain barrier opening in a large animal model using closed-loop microbubble cavitation-based feedback control of focused ultrasound sonication. Sci. Rep. 2022, 12, 16147.
- Burgess, M.T.; Apostolakis, I.; Konofagou, E.E. Power cavitation-guided blood-brain barrier opening with focused ultrasound and microbubbles. Phys. Med. Biol. 2018, 63, 065009.
- Kooiman, K.; Roovers, S.; Langeveld, S.A.G.; Kleven, R.T.; Dewitte, H.; O’Reilly, M.A.; Escoffre, J.M.; Bouakaz, A.; Verweij, M.D.; Hynynen, K.; et al. Ultrasound-Responsive Cavitation Nuclei for Therapy and Drug Delivery. Ultrasound Med. Biol. 2020, 46, 1296–1325.
- Klibanov, A.L. Preparation of targeted microbubbles: Ultrasound contrast agents for molecular imaging. Med. Biol. Eng. Comput. 2009, 47, 875–882.
- Ibsen, S.; Schutt, C.E.; Esener, S. Microbubble-mediated ultrasound therapy: A review of its potential in cancer treatment. Drug Des. Devel. Ther. 2013, 7, 375–388.
- Blomley, M.J.; Cooke, J.C.; Unger, E.C.; Monaghan, M.J.; Cosgrove, D.O.J.B. Microbubble contrast agents: A new era in ultrasound. BMJ 2001, 322, 1222–1225.
- Arvanitis, C.D.; Askoxylakis, V.; Guo, Y.; Datta, M.; Kloepper, J.; Ferraro, G.B.; Bernabeu, M.O.; Fukumura, D.; McDannold, N.; Jain, R.K. Mechanisms of enhanced drug delivery in brain metastases with focused ultrasound-induced blood-tumor barrier disruption. Proc. Natl. Acad. Sci. USA 2018, 115, E8717–E8726.
- Mainprize, T.; Lipsman, N.; Huang, Y.; Meng, Y.; Bethune, A.; Ironside, S.; Heyn, C.; Alkins, R.; Trudeau, M.; Sahgal, A.; et al. Blood-Brain Barrier Opening in Primary Brain Tumors with Non-invasive MR-Guided Focused Ultrasound: A Clinical Safety and Feasibility Study. Sci. Rep. 2019, 9, 321.
- Cai, X.; Yang, F.; Gu, N. Applications of magnetic microbubbles for theranostics. Theranostics 2012, 2, 103–112.
- Dussik, K. On the possibility of using ultrasound waves as a diagnostic aid. Neurol. Psychiat. 1942, 174, 153–168.
- Stride, E.; Edirisinghe, M. Novel microbubble preparation technologies. Soft Matter. 2008, 4, 2350–2359.
- Gramiak, R.; Shah, P.M. Echocardiography of the aortic root. Investig. Radiol. 1968, 3, 356–366.
- De Cock, I.; Zagato, E.; Braeckmans, K.; Luan, Y.; de Jong, N.; De Smedt, S.C.; Lentacker, I. Ultrasound and microbubble mediated drug delivery: Acoustic pressure as determinant for uptake via membrane pores or endocytosis. J. Control. Release 2015, 197, 20–28.
- Teng, W.; Huneiti, Z.; Machowski, W.; Evans, J.; Edirisinghe, M.; Balachandran, W. Towards particle-by-particle deposition of ceramics using electrostatic atomization. J. Mater. Sci. Lett. 1997, 16, 1017–1019.
- Tinkov, S.; Bekeredjian, R.; Winter, G.; Coester, C. Microbubbles as ultrasound triggered drug carriers. J. Pharm. Sci. 2009, 98, 1935–1961.
- Surya, V.; Manaz, M.; Sharon, P.; Shanmugam, K. Ultrasound-Targeted Microbubble Destruction (UTMD): Targeted Nanodrug Delivery in Cancer. BOHR Int. J. Cancer Res. 2022, 1, 13–15.
- He, J.; Liu, Z.; Zhu, X.; Xia, H.; Gao, H.; Lu, J. Ultrasonic Microbubble Cavitation Enhanced Tissue Permeability and Drug Diffusion in Solid Tumor Therapy. Pharmaceutics 2022, 14, 1642.
- Yang, F.Y.; Wang, H.E.; Lin, G.L.; Teng, M.C.; Lin, H.H.; Wong, T.T.; Liu, R.S. Micro-SPECT/CT-based pharmacokinetic analysis of 99mTc-diethylenetriaminepentaacetic acid in rats with blood-brain barrier disruption induced by focused ultrasound. J. Nucl. Med. 2011, 52, 478–484.
- Tung, Y.S.; Vlachos, F.; Feshitan, J.A.; Borden, M.A.; Konofagou, E.E. The mechanism of interaction between focused ultrasound and microbubbles in blood-brain barrier opening in mice. J. Acoust. Soc. Am. 2011, 130, 3059–3067.
- Singh, B.; Shukla, N.; Cho, C.-H.; Kim, B.S.; Park, M.-H.; Kim, K. Effect and application of micro- and nanobubbles in water purification. Toxicol. Environ. Health Sci. 2021, 13, 9–16.
- Kamaev, P.P.; Hutcheson, J.D.; Wilson, M.L.; Prausnitz, M.R. Quantification of Optison bubble size and lifetime during sonication dominant role of secondary cavitation bubbles causing acoustic bioeffects. J. Acoust. Soc. Am. 2004, 115, 1818–1825.
- Azmin, M.; Harfield, C.; Ahmad, Z.; Edirisinghe, M.; Stride, E. How do microbubbles and ultrasound interact? Basic physical, dynamic and engineering principles. Curr. Pharm. Design 2012, 18, 2118–2134.
- Jangjou, A.; Meisami, A.H.; Jamali, K.; Niakan, M.H.; Abbasi, M.; Shafiee, M.; Salehi, M.; Hosseinzadeh, A.; Amani, A.M.; Vaez, A. The promising shadow of microbubble over medical sciences: From fighting wide scope of prevalence disease to cancer eradication. J. Biomed. Sci. 2021, 28, 49.
- Abrahao, A.; Meng, Y.; Llinas, M.; Huang, Y.; Hamani, C.; Mainprize, T.; Aubert, I.; Heyn, C.; Black, S.E.; Hynynen, K.; et al. First-in-human trial of blood-brain barrier opening in amyotrophic lateral sclerosis using MR-guided focused ultrasound. Nat. Commun. 2019, 10, 4373.
- Zhan, W. Effects of Focused-Ultrasound-and-Microbubble-Induced Blood-Brain Barrier Disruption on Drug Transport under Liposome-Mediated Delivery in Brain Tumour: A Pilot Numerical Simulation Study. Pharmaceutics 2020, 12, 69.
- Lipsman, N.; Meng, Y.; Bethune, A.J.; Huang, Y.; Lam, B.; Masellis, M.; Herrmann, N.; Heyn, C.; Aubert, I.; Boutet, A.; et al. Blood-brain barrier opening in Alzheimer’s disease using MR-guided focused ultrasound. Nat. Commun. 2018, 9, 2336.
- Stride, E.; Edirisinghe, M. Novel preparation techniques for controlling microbubble uniformity: A comparison. Med. Biol. Eng. Comput. 2009, 47, 883–892.
- Roovers, S.; Segers, T.; Lajoinie, G.; Deprez, J.; Versluis, M.; De Smedt, S.C.; Lentacker, I. The Role of Ultrasound-Driven Microbubble Dynamics in Drug Delivery: From Microbubble Fundamentals to Clinical Translation. Langmuir 2019, 35, 10173–10191.
- Sheeran, P.S.; Matsunaga, T.O.; Dayton, P.A. Phase change events of volatile liquid perfluorocarbon contrast agents produce unique acoustic signatures. Phys. Med. Biol. 2014, 59, 379–401.
- Su, C.; Ren, X.; Nie, F.; Li, T.; Lv, W.; Li, H.; Zhang, Y. Current advances in ultrasound-combined nanobubbles for cancer-targeted therapy: A review of the current status and future perspectives. RSC Adv. 2021, 11, 12915–12928.
- Stride, E.; Segers, T.; Lajoinie, G.; Cherkaoui, S.; Bettinger, T.; Versluis, M.; Borden, M. Microbubble agents: New directions. Ultrasound Med. Biol. 2020, 46, 1326–1343.
- Abou-Saleh, R.H.; McLaughlan, J.R.; Bushby, R.J.; Johnson, B.R.; Freear, S.; Evans, S.D.; Thomson, N.H. Molecular Effects of Glycerol on Lipid Monolayers at the Gas-Liquid Interface: Impact on Microbubble Physical and Mechanical Properties. Langmuir 2019, 35, 10097–10105.
- Tran, W.T.; Iradji, S.; Sofroni, E.; Giles, A.; Eddy, D.; Czarnota, G.J. Microbubble and ultrasound radioenhancement of bladder cancer. Br. J. Cancer 2012, 107, 469–476.
- Wu, T.; Huang, C.; Yao, Y.; Du, Z.; Liu, Z. Suicide Gene Delivery System Mediated by Ultrasound-Targeted Microbubble Destruction: A Promising Strategy for Cancer Therapy. Hum. Gene. Ther. 2022, 33, 1246–1259.
- Fan, C.H.; Wang, T.W.; Hsieh, Y.K.; Wang, C.F.; Gao, Z.; Kim, A.; Nagasaki, Y.; Yeh, C.K. Enhancing Boron Uptake in Brain Glioma by a Boron-Polymer/Microbubble Complex with Focused Ultrasound. ACS Appl. Mater. Interfaces 2019, 11, 11144–11156.
- Schwendener, R.A.; Schott, H. Liposome Formulations of Hydrophobic Drugs. Methods Mol. Biol. 2017, 1522, 73–82.
- Prasad, C.; Banerjee, R. Ultrasound-Triggered Spatiotemporal Delivery of Topotecan and Curcumin as Combination Therapy for Cancer. J. Pharmacol. Exp. Ther. 2019, 370, 876–893.
- Al-Jawadi, S.; Thakur, S.S. Ultrasound-responsive lipid microbubbles for drug delivery: A review of preparation techniques to optimise formulation size, stability and drug loading. Int. J. Pharm. 2020, 585, 119559.
- Chang, E.L.; Ting, C.Y.; Hsu, P.H.; Lin, Y.C.; Liao, E.C.; Huang, C.Y.; Chang, Y.C.; Chan, H.L.; Chiang, C.S.; Liu, H.L.; et al. Angiogenesis-targeting microbubbles combined with ultrasound-mediated gene therapy in brain tumors. J. Control. Release 2017, 255, 164–175.
- Fan, C.H.; Cheng, Y.H.; Ting, C.Y.; Ho, Y.J.; Hsu, P.H.; Liu, H.L.; Yeh, C.K. Ultrasound/Magnetic Targeting with SPIO-DOX-Microbubble Complex for Image-Guided Drug Delivery in Brain Tumors. Theranostics 2016, 6, 1542–1556.
- Ting, C.Y.; Fan, C.H.; Liu, H.L.; Huang, C.Y.; Hsieh, H.Y.; Yen, T.C.; Wei, K.C.; Yeh, C.K. Concurrent blood-brain barrier opening and local drug delivery using drug-carrying microbubbles and focused ultrasound for brain glioma treatment. Biomaterials 2012, 33, 704–712.
- Fan, C.H.; Ting, C.Y.; Liu, H.L.; Huang, C.Y.; Hsieh, H.Y.; Yen, T.C.; Wei, K.C.; Yeh, C.K. Antiangiogenic-targeting drug-loaded microbubbles combined with focused ultrasound for glioma treatment. Biomaterials 2013, 34, 2142–2155.
- Ha, S.W.; Hwang, K.; Jin, J.; Cho, A.S.; Kim, T.Y.; Hwang, S.I.; Lee, H.J.; Kim, C.Y. Ultrasound-sensitizing nanoparticle complex for overcoming the blood-brain barrier: An effective drug delivery system. Int. J. Nanomed. 2019, 14, 3743–3752.
- Zhao, G.; Huang, Q.; Wang, F.; Zhang, X.; Hu, J.; Tan, Y.; Huang, N.; Wang, Z.; Wang, Z.; Cheng, Y. Targeted shRNA-loaded liposome complex combined with focused ultrasound for blood brain barrier disruption and suppressing glioma growth. Cancer Lett. 2018, 418, 147–158.
- Yang, F.Y.; Lin, G.L.; Horng, S.C.; Chang, T.K.; Wu, S.Y.; Wong, T.T.; Wang, H.E. Pulsed high-intensity focused ultrasound enhances the relative permeability of the blood-tumor barrier in a glioma-bearing rat model. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2011, 58, 964–970.
- Dong, L.; Li, N.; Wei, X.; Wang, Y.; Chang, L.; Wu, H.; Song, L.; Guo, K.; Chang, Y.; Yin, Y.; et al. A Gambogic Acid-Loaded Delivery System Mediated by Ultrasound-Targeted Microbubble Destruction: A Promising Therapy Method for Malignant Cerebral Glioma. Int. J. Nanomed. 2022, 17, 2001–2017.
- Park, S.H.; Yoon, Y.I.; Moon, H.; Lee, G.H.; Lee, B.H.; Yoon, T.J.; Lee, H.J. Development of a novel microbubble-liposome complex conjugated with peptide ligands targeting IL4R on brain tumor cells. Oncol. Rep. 2016, 36, 131–136.
