Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biotechnology & Applied Microbiology
Hydrogen is widely considered as the fuel of the future. Due to the challenges present during hydrogen production using conventional processes and technologies, additional methods must be considered, like the use of microorganisms. One of the most promising technologies is dark fermentation, a process where microorganisms are utilized to produce hydrogen from biomass.
- biological hydrogen
- biowaste
- storage
1. Introduction
There is an increasing energy demand in the modern world. It is expected to be even more pronounced in the future as it is imposed by modern lifestyle and economic development [1]. However, natural resources for energy production are limited [2]. The major problem is posed by the use of non-renewable fossil fuel energy sources and the technologies used for production, which are direct polluters of the environment, leading to the generation of greenhouse gases and global warming [3,4,5].
The development of alternative, clean energy sources represents the right path toward solving the energy problems of humankind [6]. A practical alternative to fossil fuels is the utilization of hydrogen, which has been under discussion for a long time. It is included in the plans of countries in the European Union and beyond [7]. In line with the European Green Deal of 2020, the European Commission presented the Hydrogen Strategy for a Climate-Neutral Europe by 2050. Hydrogen is highlighted as one of the key levers for a successful energy transition and the European strategy for integrating energy systems [8]. The global hydrogen production in 2021 was about 90 megatons per year (Mt/y). However, most hydrogen is produced from fossil fuels, and less than 1% comes from renewable resources and microorganisms [9].
Hydrogen (H2) is the lightest element in the periodic table of elements and the most abundant chemical element in the universe [10]. It is a colorless, odorless, non-toxic, and highly flammable gas at standard pressure and density. It is difficult to find in its pure molecular form as it is lighter than air and rises from the atmosphere [11]. In the energy industry, hydrogen is nominated with a color designating the primary sources of energy used for hydrogen generation.
2. Hydrogen Production from Biomass
First-generation biomass mainly consists of Lignocellulosic biomass with high starch and sugar content, such as sweet sorghum, sugar beet, potato, wheat, pumpkin, and oilseed rape, as well as the residues and byproducts of their processing, which are traditionally utilized for food and feed [75]. Second-generation biomass is mainly represented by lignocellulosic biomass with low commercial value but high abundance. Third-generation biohydrogen production is derived from algal biomass rich in polysaccharides [76]. Algae have the ability to grow rapidly compared to other biomasses and sequester large amounts of CO2. In addition, they can contain a low amount of lignin and lignin oligomers [77,78], which makes them easy to hydrolyze [79]. The fourth-generation biomass feedstock comprises genetically modified organisms to improve biohydrogen production processes [80]. Second and third-generation biomasses, after genetic modification, become part of the fourth-generation feedstock. Genetic/metabolic modifications in microorganisms capable of contributing to the development of new technologies are achieved through genetic engineering or nanotechnology [35,81].
2.1. Lignocellulosic Biomass and Crop Residues Containing Sugars
Lignocellulosic biomass is considered “the most abundant organic component of the biosphere”, with an annual production of 1–5·1013 kg, which is considered an attractive and cheap substrate for the production of biofuels [82,83]. It is the most prevalent in nature and is present as hardwood, softwood, grasses, and agricultural residues. The estimated global annual yields of lignocellulosic biomass residues are more than 220 Bt [84], which is equivalent to 60–80 Bt of crude oil [15].
Based on dry matter composition, lignocellulosic biomass primarily consists of cellulose (40–60%), hemicellulose (20–40%), and lignin (10–25%) [85]. Cellulose and hemicellulose can be enzymatically hydrolyzed into smaller sugar molecules [86]. They mainly contain glucose and xylose, readily available for microorganisms that can effectively ferment glucose, and xylose are for bio-hydrogen production [87] (Figure 1).
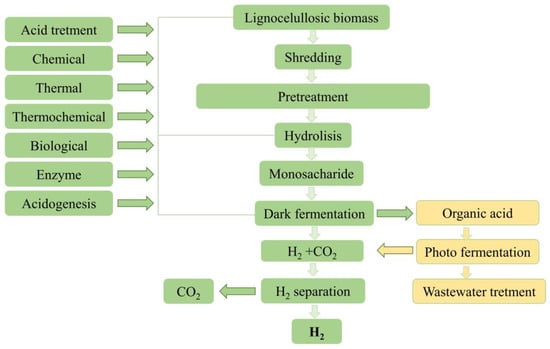
Figure 1. Process for obtaining hydrogen from lignocellulosic biomass.
In order to improve substrate hydrolysis and facilitate biological hydrogen production through dark fermentation in reactors, pretreatment of the lignocellulosic biomass matrix is used to release cellulose molecules into solution, breaking the crystal structure of cellulose and helping depolymerization [87]. Pretreatment can also be applied to the inoculum to enrich hydrogen-producing bacteria and inhibit hydrogen-consuming bacteria [88]. Mixed cultures have an advantage over pure cultures because they allow easier control of the process and higher substrate efficiency, but pretreatment should be performed in this case. Acid or alkaline treatment, thermal shock, aeration, chemical inhibition, or inhibition with long-chain fatty acids can be used to increase hydrogen production by dark fermentation. Pretreatment aims to suppress the growth of hydrogen consumers that do not form spores. Spore-forming hydrogen-producing bacteria survive this pretreatment [89].
Hydrogen can be produced from lignocellulosic biomass through dark fermentation with a yield close to the maximum theoretical yield of 4 moles of hydrogen per mole of hexose [15]. It is worth mentioning that a wide range of monosaccharides, disaccharides, and polysaccharides (including cellulose) can be used for hydrogen production [90].
Caldicellulosiruptor saccharolyticus is an example of an interesting bacterium for bio-hydrogen production. C. saccharolyticus is a thermophile, strictly anaerobic, Gram-positive, cellulolytic bacterium bio-hydrogen producer [90]. Genome analysis revealed that C. saccharolyticus has a high ability to hydrolyze polysaccharides such as cellulose, hemicellulose, pectin, and starch, along with a large number of ABC transporters for the uptake of monomeric and oligomeric sugars [91]. Catabolic pathways have been identified for a range of sugars, including rhamnose, fucose, arabinose, glucuronic acid, fructose, and galactose, which lead to the production of NADH and reduced ferredoxin. NADH and reduced ferredoxin later use two different hydrogenases to form hydrogen [92]. Whole-genome transcriptome analysis revealed significant regulation of the glycolytic pathway and ABC-type sugar transporters during growth on glucose and xylose, indicating that C. saccharolyticus coferments these sugars without repression based on glucose catabolite [82]. In addition, C. saccharolyticus is an extreme thermophile. It is known that H2 yields are higher in thermophilic than in mesophilic conditions, although the volumetric productivity is reversed [56]. Thus, transferring the metabolic pathway of C. saccharolyticus to a mesophilic bacterium would be advantageous. The role of genetic engineering in cellulolytic microorganisms, in fact, is significant and enables the redirection of metabolic pathways toward maximum hydrogen production [93]. For example, introducing mutations in mesophilic E. coli by inactivating the lactate dehydrogenase gene (ldhA) increases hydrogen production by 20–45% [94].
To increase H2 production in fermentation systems, modifications to metabolic engineering as the introduction/overexpression of genes (cellulases, hemicellulases, and lignases), can be used to increase the availability of carbohydrates for the cell, overexpression of enzymes that produce H2, and disruption of metabolic pathways that compete for reductive equivalents [15].
2.2. Wastewaters
Wastewater, particularly those from industrial facilities, presents a promising avenue for water treatment companies to apply methods of wastewater recycling to produce green hydrogen directly using microorganisms [95]. Wastewater consists of 70% organic compounds and 30% inorganic compounds, with organic compounds primarily being carbohydrates, fats, and proteins, which can be utilized through dark fermentation for hydrogen production [96]. In dark fermentation methods, bacteria such as Clostridium thermocellum break down organic materials and produce hydrogen [32].
The wastewater microbial consortium contains a large number of bacteria, some of which inhibit hydrogen production (i.e., hydrogenophilic methanogenesis) through consumption (homo-acetogens and methanogens) [95]. Therefore, for optimal hydrogen production, the activity of inhibitory microorganisms is suppressed or killed, most commonly through pre-heating of the inoculum [97] or, in a more cheap way, optimizing the parameters processes as the initial pH [98].
Potentially, wastewater constitutes readily accessible and inexpensive biomass from which hydrogen can be produced [99]. This would reduce the negative impact of wastewater treatment on the environment while increasing hydrogen production, thus making wastewater a fuel of the future [100]. Essentially, green hydrogen from wastewater has tremendous potential for further development [101].
Organic wastewater contains a potentially high energy content, with each kg of chemical oxygen demand producing about 1.4 × 107 kg of metabolic heat, which has immense practical significance [41,102,103]. It has been noted that the energy contained in wastewater is 9.3 times greater than the energy consumed for its treatment [104]. If 10% of the energy can be utilized, it could power the wastewater treatment facility. As a result, energy extraction from organic waste is undoubtedly critical for developing low-carbon “energy saving and emissions reduction” models and for developing renewable energy [41].
3. Hydrogen Management
It would be ideal to use bio-hydrogen on the production site [105]. However, these are mainly energy generation systems by combustion, and the produced energy is transferred to other systems through a heat exchanger: biological, technical, and physical processes [31]. In this way, energy losses are minimized, and the costs of preparation for transport, transport, and storage, as well as the costs of re-releasing pure hydrogen as a fuel at the destination, are avoided [106].
However, quantities of produced gas from 100,000 m3 to several million cubic meters must be stored in natural or technically advanced geological formations, ensuring the conditions of impermeability, preservation of the purity of stored hydrogen from bacterial, organic, and inorganic pollution, and the possibility of increasing storage space [107].
3.1. Hydrogen Storage
The final choice of the method and form of hydrogen handling—storage depends on its final use, the form of energy, and the energy conversion method [108].
3.1.1. Natural Caves and Salt Mine Caves
The walls of such caves ensure the impermeability and preservation of the storage gas’s purity [109]. Such storage facilities are already used in the USA and the UK [110]. Such a form of storage is considered the most economical option and the safest gas storage facility that is well protected from external influences (terrorist attacks, fires, and military actions) [110]. The capacities of such caves are around one million cubic meters [111]. In general, pure hydrogen is in the form of fuel cells ready for technological application (vehicles, etc.) [112].
3.1.2. Underground Hydrogen Storage in a Gas Mixture
Besides its nascent form, hydrogen can be stored in depleted natural gas deposits (natural gas: a mixture of methane and higher homologs) or a mixture with methane, CO2, and CO (synthetic gas) [113], as well as in a mixture with city gas (methane, CO) [114].
All of these forms of stored gas can be utilized in gas turbines or fuel cells to produce electrical energy [115]. Such mixtures can be stored in salt caverns and aquifers (so-called depleted geological environments saturated with free subterranean waters) [116]. They form in water-impermeable rocks as well as in natural gas storage facilities (hydrogen content of 5–15%) [108].
Hydrogen must be separated from the mixture with natural gas in pure form for further use [117]. This can be achieved through membrane filtration, adsorption, and electrochemical separation [118].
3.1.3. Special Cases of Hydrogen Storage in Subsurface Reactors for the Methanation Process
In underground storage sites of natural gas and aquifers, hydrogen and carbon dioxide are subjected to hydrogenotrophic methanogenic bacteria that, through the process of methanization, create methane [119]. This process takes place at low temperatures, which is more acceptable than some high-temperature processes with catalysts [120].
3.2. Hydrogen Transport
Hydrogen transport can be considered a special form of hydrogen storage [121]. Long-distance transportation is carried out based on the technical principles of natural gas transport [122]. Hydrogen can be transported as a liquefied gas or as hydrogen converted into ammonia, methanol, or another transportable fluid. However, additional costs such as “energy loss” are incurred [123].
The most favorable cost-to-output ratio for hydrogen transport is transporting in liquefied form or the form of ammonia, with the possibility of hydrogen production via cracking at the consumption site [124]. However, the most significant drawbacks of these technologies are the costs of liquefaction and cracking, and the more promising technology is liquefaction [125]. Currently, efforts are underway to develop technologies that reduce costs when hydrogen is used as an energy source rather than a raw material in the form of ammonia [126].
Hydrogen can also be transported and stored using other organic carriers such as methanol and methylcyclohexane [127]. In hydrogen transport systems, such as pipelines, the phenomenon of diffusion occurs in the material the pipeline is made of [128]. This phenomenon is known as “hydrogen brittleness” and manifests as a reduction in the mechanical properties of pipelines. The degree of hydrogen brittleness depends on the operation regime of the pipeline (fluctuation of pressures, the type of material the pipeline is made of) [129]. Therefore, processes for handling hydrogen after production in terms of transport and storage represent an enormous challenge in finding the most efficient, safe, and cost-effective methods [130]. However, given the growing commitment to hydrogen as an energy source of the future, we are confident that optimal solutions will also be found for handling hydrogen [105].
3.3. Hydrogen Economics
According to Kayfeci et al., hydrogen production can cost between 1.25 USD/kg and 23.27 USD/kg, respectively [126,131]. The choice of the production method, in this case, is impacting the H2 production costs, where fossil fuel-based technologies tend to have relatively low costs, 1.34–2.27 USD/kg, while solar-based technologies (PV, thermal, solar thermolysis, and photo-thermolysis) tend to be with the highest cost varying between 5.78–23.27 USD/kg) [132]. Among the most competitive options are indirect bio-photolysis, with a cost of 1.42 USD/kg, and direct bio-photolysis, with a cost of 2.13 USD/kg [24]. The dark fermentation method is estimated to cost 2.57 USD/kg, which is in the same range as nuclear thermolysis option 2.17–2.63 USD/kg, while nuclear electrolysis is estimated to have costs 4.15–7.00 USD/kg, respectively [42] (Table 1).
| Process/Method | Cost (USD/kg) |
|---|---|
| Dark fermentation | 2.57 |
| Photo-fermentation | 2.83 |
| Biomass pyrolysis | 1.25–2.20 |
| Biomass gasification | 1.77–2.05 |
| Direct biophotolysis | 2.13 |
| Indirect biophotolysis | 1.42 |
| Dark fermentation | 2.57 |
| Photo-fermentation | 2.83 |
This entry is adapted from the peer-reviewed paper 10.3390/en16083321
This entry is offline, you can click here to edit this entry!
