Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Non-alcoholic fatty liver disease (NAFLD) is the most common cause of abnormal liver function tests worldwide, with an estimated prevalence ranging between 19–46% in the general population. Given the high prevalence and severity of NAFLD, especially in high-risk populations (i.e., patients with type-2 diabetes mellitus and/or obesity), there is a major interest in early detection of the disease in primary care.
- non-alcoholic fatty liver disease
- screening
- primary care
1. Definition and Epidemiology of NAFLD
Non-alcoholic fatty liver disease (NAFLD) is the most common cause of abnormal liver function tests worldwide, with a global estimated prevalence of 30% [1]. NAFLD encompasses a spectrum of pathological disorders characterised by macro-vesicular fat accumulation (steatosis, non-alcoholic fatty liver, NAFL) with or without hepatocellular injury and/or inflammation (non-alcoholic steato-hepatitis, NASH) and a variable degree of fibrosis up to cirrhosis [2][3].
Overall, NAFLD prevalence is particularly high in those with metabolic syndrome, i.e., a combination of central obesity, insulin resistance, type 2 diabetes mellitus (T2DM), hypertension and dyslipidaemia [4]. According to tertiary care studies, more than 50% of the patients with T2DM have NAFLD [4]. Similarly, the prevalence of NAFLD is as high as 45% among those with increased body mass index (BMI > 30 kg/m2) and up to 90% among those undergoing bariatric surgery (BMI > 35 kg/m2) [5]. Mirroring the epidemic of metabolic syndrome, the prevalence of NAFLD is constantly increasing in the general population, increasing from 33% in 2005 to 59.1% in 2010, and in similar fashion, the prevalence of NASH increasing from 15% to 25% [6]. The total NAFLD population in 2015 was estimated at 83.1 million cases, which is projected to increase by 21% to 100.9 million cases by 2030 [7]. Furthermore, NAFLD/NASH has become the fastest growing indication for liver transplantation in the USA [8].
2. Pathogenesis of NAFLD
Far from the old concept of being a dichotomic disease, NAFLD is now considered to be a dynamic disease with a wide spectrum of disease activity within different stages of simple steatosis or NASH [9]. Insulin resistance plays a crucial role in the development and progression of liver disease, as this stimulates de novo lipogenesis and is associated with impaired lipolysis, resulting in an increased flux of fatty acid to the liver [10]. Of note, hepatic triglyceride storage is not harmful per se. Nevertheless, when the hepatic capacity of using, storing, and exporting free fatty acids becomes saturated, lipotoxicity may occur within the liver. Lipotoxicity is thought to be the crucial driver for the development and progression of hepatocellular injury, inflammation, hepatic stellate cell activation and extracellular matrix deposition, leading to fibrosis progression [11]. Overall, a status of insulin resistance also drives a dysfunctional adipose tissue, which produces metabolically active cytokines and initiates an inflammatory cascade [12] (Figure 1).
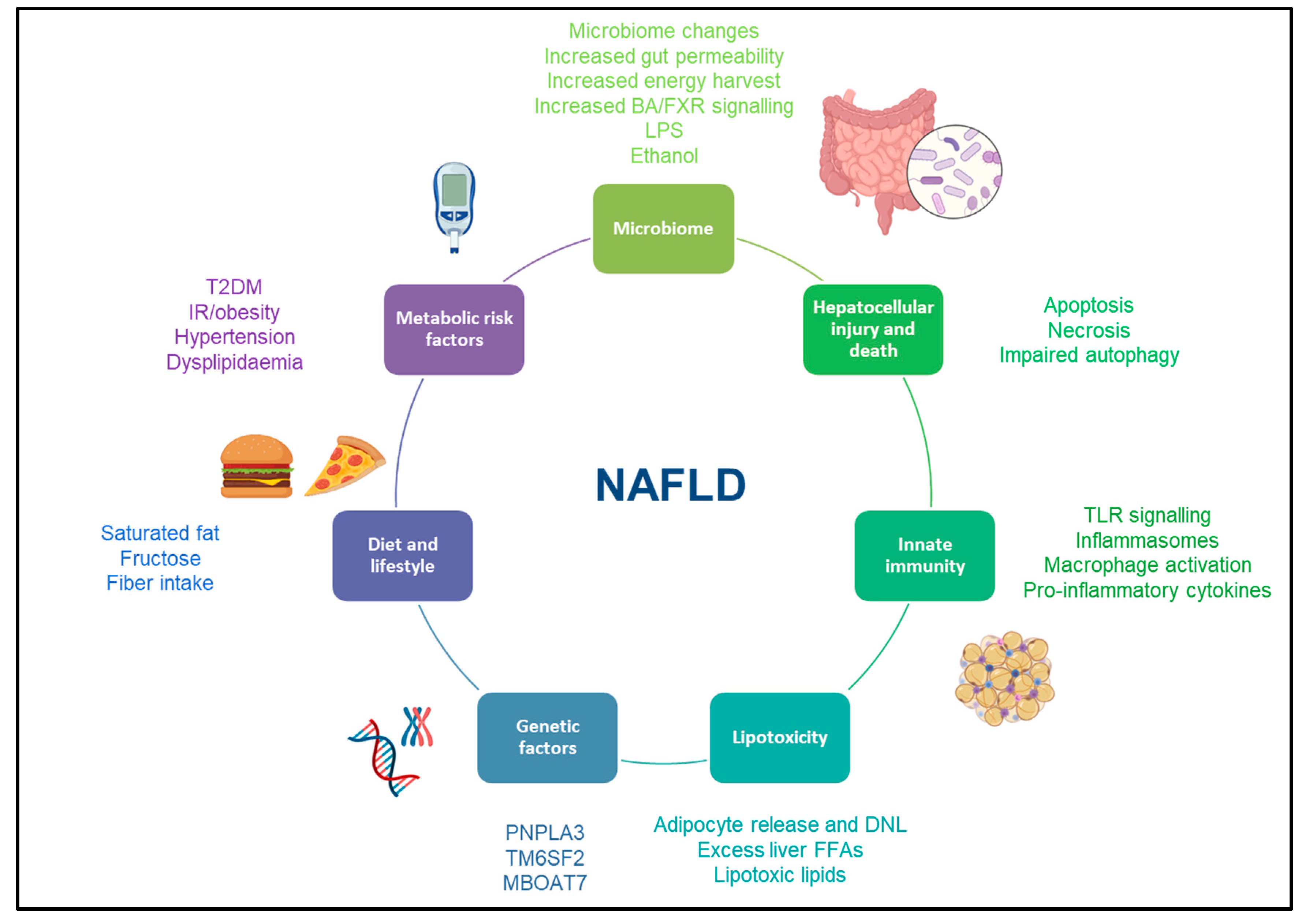
Figure 1. Risk factors for development and progression of NAFLD. This figure illustrates the most common risk factors associated with the development and progression of NAFLD. Abbreviations: NAFLD: non-alcoholic fatty liver disease, BA: bile acid, FXR: farnesoid X receptor, LPS: lipopolysaccharide, TLR: toll-like receptor, DNL: de novo lipogenesis, T2DM: type 2 diabetes mellitus, IR: insulin resistance, PNPLA3: patatin-like phospholipase domain containing protein 3, TM6SF2: Transmembrane 6 superfamily member 2, MBOAT7: membrane-bound O-acyltransferase domain containing 7.
3. Screening for NAFLD in Primary Care: Current Recommendations
Given the high prevalence and severity of NAFLD in those with metabolic syndrome and type-2 diabetes, there is an expected large burden of undiagnosed NAFLD with advanced fibrosis in the community, and—as such—a major interest in early detection of the disease in primary care [13]. Furthermore, as there is no licensed treatment for the disease, early detection of fibrosis is of the utmost importance in this population. For instance, in the United States, it has been estimated that up to 9 million diabetic patients have NASH, while 4 million are at risk for advanced fibrosis [14]. Similar evidence has been made available for those suffering from obesity [15].
The latest European guidelines recommend screening NAFLD in high-risk populations (i.e., patients with metabolic syndrome) following a two-tier system (Figure 2). Specifically, it is recommended that patients should be stratified using FIB-4 and/or ELF in primary care, followed in sequence by TE in a specialist setting [13]. The most recent guidelines from the America Association for the study of the liver diseases (AASLD) suggest yearly testing with FIB-4 for diabetics and those with at least two components of metabolic syndrome, whilst not recommending screening for NAFLD in the general population [16]. Interestingly, the latest UK NICE guidelines [17] recommend screening for NAFLD subjects with T2DM and metabolic syndrome, including LFTs and/or ultrasound. Similar recommendations are made in the guidelines from the Asian Pacific Association [18] and from the Latin American Association [19] for the study of the liver. However, LFTs assessment is not sufficient alone for screening NAFLD, since it is well established that NASH and significant fibrosis can occur in patients with normal range LFTs [20][21]. Furthermore, ultrasound has low reproducibility and was not designed to stage disease severity [13].
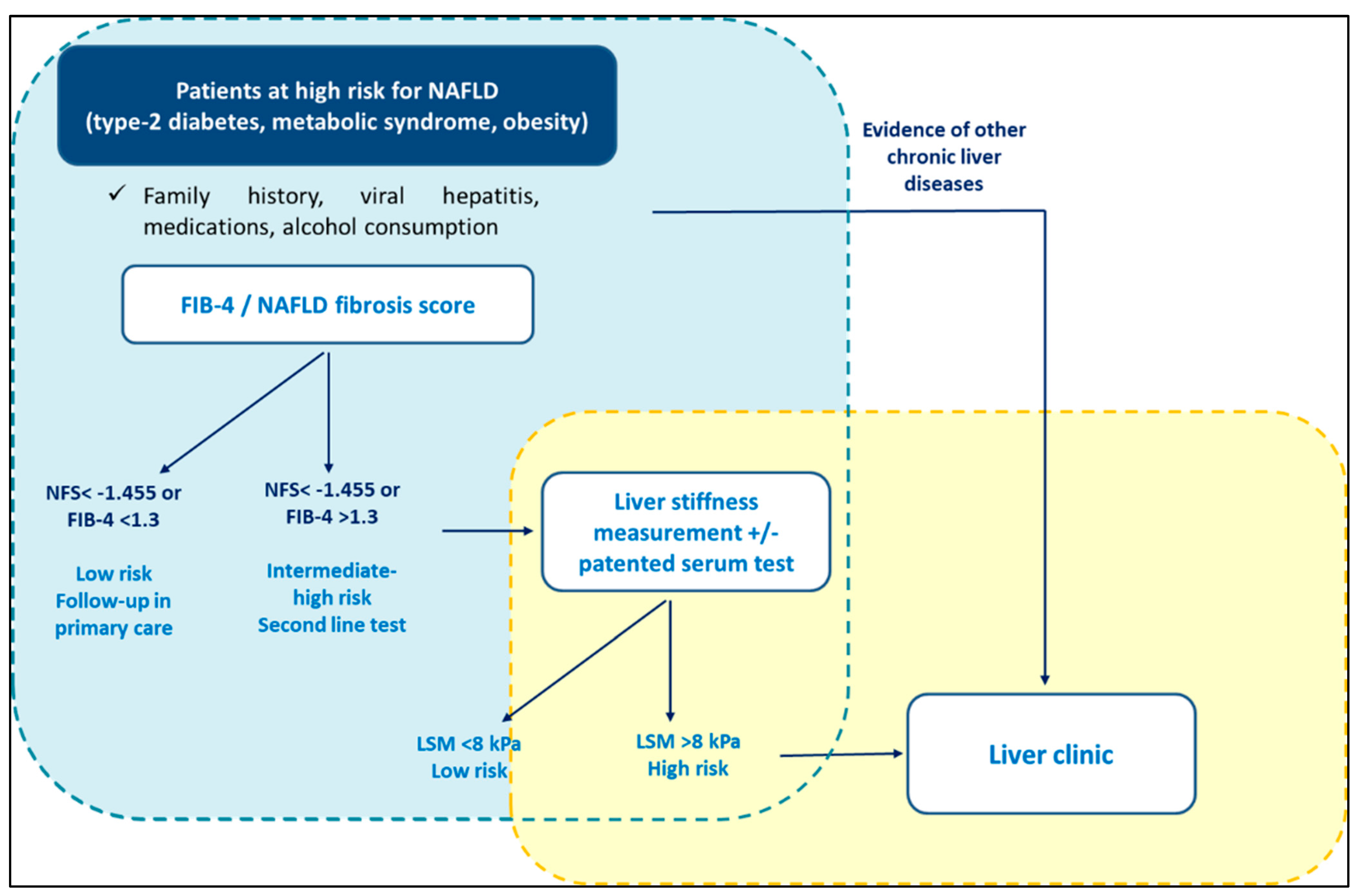
Figure 2. Screening for NAFLD in primary care. International guidelines recommend screening for NAFLD in high-risk populations (i.e., patients with metabolic syndrome) following a two-tier system.
Unfortunately, there is a substantial lack of awareness among policy makers outside the hepatology community. For instance, current diabetes and obesity management guidelines do not advise for NAFLD screening in the respective populations [22]. Nevertheless, the American Association of Clinical Endocrinology has now published a clinical practice guideline on the management of NAFLD in primary care and endocrinology settings, opening the door to future joint position papers across different specialties [23]. Such guidelines highlight that patients at high risk of NAFLD do not require an abdominal ultrasound to diagnose hepatic steatosis; it is recommended to move directly to risk stratification. Finally, European guidelines on obesity care in patients with chronic GI conditions, despite recognising that obese patients should be screened for NAFLD, do not advise fibrosis risk stratification [24].
4. Screening for NAFLD in Primary Care: Limitations
Although screening for NAFLD in high-risk populations has been supported by EASL and AASLD guidelines, a consensus on the cost-effectiveness of screening has not yet been reached. Corey and colleagues performed a simulation to compare quality-adjusted life years (QALYs) between screening with liver biopsy and non-screening, including pioglitazone as therapeutical option. The authors reported that NASH screening could have been cost-effective if superior treatment had been made available at the time of the model [25]. A recent paper suggested that for a pharmacological intervention to be cost-effective in the NAFLD fibrosis population, the annual drug cost should not exceed $12,000 per patient [26]. Several studies tried to analyse the cost-effectiveness of screening by factoring in the effect of early detection in slowing disease progression rather than the therapeutical effect of a new pharmacological agent. A recent cost-utility analysis also demonstrated that screening patients with T2DM with US and LFTs, followed by non-invasive tests, was more cost-effective than not screening [27]. Interestingly, a UK-based study comparing risk stratification using TE vs. standard of care proved to be cost-effective in the general population [28]. Similarly, in a study conducted in the US health system, screening for NAFLD cirrhosis with FIB-4, followed by TE and liver biopsy, was more effective than FIB-4 followed by MRE [29]. Another study demonstrated that FIB-4 followed by share wave elastography was the most effective and least costly strategy in the community [30]. Nevertheless, the lack of licensed pharmacological treatment still represents an important limitation to establishing the cost-effectiveness of screening in this population. Furthermore, a recent metanalysis also pointed out how the currently used health economic models are associated with limitations, primarily driven by a lack of NASH-specific data [31].
Primary care clinicians play an essential role in identifying patients with NAFLD who are at risk of significant liver disease [13]. In this sense, the Lancet commission on liver disease identified the need for streamlined diagnostic pathways for screening people with NAFLD as a priority area to defeat liver disease [32]. However, an important limitation to screening pathways of NAFLD is an overall low awareness among primary care clinicians, possibly as the result of gaps in knowledge as well as lack of awareness of relevant practice guidelines. In a survey study, over 40% of general practitioners (GPs) were not familiar with clinical published guidelines for NAFLD management [33]. Moreover, GPs were more likely to screen low-risk patients while neglecting patients at high risk for liver fibrosis. Again, this phenomenon has been attributed to the misconception that LFTs may reflect disease severity. On a similar note, a UK-based qualitative study demonstrated that the diagnosis and management of NAFLD is perceived as a great challenge by GPs [34][35]. Overall, less than 3% of patients with elevated FIB-4 are currently referred to the specialist setting for further investigations [36], with GPs not perceiving NAFLD as a priority in their clinical activities [37].
Low standardisation of the screening protocols also represents an important limitation to screening NAFLD in the community. Several studies have highlighted a significant gap between guidelines and real-life clinical approaches, not only across different continents [38] but also within Europe [39]. Such inconsistency translates into a lack of clarity for primary care physicians. The use of an automated calculator for NITs as well as easier access to second-line non-invasive tests have been identified as possible strategies to overcome current barriers to screening [37]. Clear guidance on the groups to be screened and the patients to be referred for further tests also appears to be lacking. A previous study carried out in Scotland demonstrated how an algorithm for analysis of abnormal LFTs was found to correctly (in 91.3% of cases) stratify patients for referral to specialist investigation [40]. A similar approach could be potentially useful for identifying those at higher risk of fibrosis from NAFLD. Furthermore, NAFLD screening could be embedded in the routine clinical management of high-risk populations in primary care, such as those with type-2 diabetes [37].
Along with developing cost-effective screening for NAFLD in primary care, future work should also focus on education regarding high-risk stratification, providing easy-to-use tools and building awareness among primary care physicians.
5. Screening for NAFLD in Primary Care: Beware of the Spectrum Effect
Ideally, screening tests should be derived from a cohort that mirrors the target population so that spectrum biases can be minimised [41]. From an epidemiological perspective, the spectrum effect describes the variation in the diagnostic performance of predictive tests when applied to populations with different disease prevalence. Due to the spectrum effect, NITs will have lower sensitivity and higher specificity in populations with lower disease prevalence. On the other hand, in secondary/tertiary care settings (higher disease prevalence), the positive predictive value will be higher, as the probability of observing true positive cases is higher a priori. Among others, NITs based on blood tests and/or on a combination of clinical features seem to be particularly affected by the spectrum bias.
Notably, most of the NITs used for NAFLD screening were historically developed and validated in secondary or tertiary care settings. Their performance in primary care is largely unknown. Specifically, FIB-4 was developed from a cohort of patients with biopsy-proven chronic hepatitis C [42], while the NAFLD fibrosis score and the ELF were developed in a cohort of patients with biopsy-proven NAFLD [43][44]. Of note, in the pre-elastography era, the average biopsy patient was selected based on clinical parameters, mainly on LFTs. It is therefore not surprising that these cohorts were characterised by older age and elevated LFTs, despite these not necessarily being a good marker of the disease severity [21]. It is therefore expected that FIB-4, NAFLD fibrosis and ELF score captures the phenotype of patients referred to specialist clinics.
The EASL algorithm for NAFLD screening has recently been validated into a tertiary care cohort, despite primary care being the main target for the pathway [45]. Interestingly, a recent real-life study showed that up to two-thirds of the new referrals to hepatology clinics are discharged after their first assessment, suggesting that current risk stratification needs optimisation [46]. Furthermore, there is emerging evidence suggesting that FIB-4 accuracy is much lower in the community [47], especially when used to assess young patients with normal liver function tests [47][48]. Moreover, a recent metanalysis also demonstrated that ELF performance is not consistent across studies [49], suggesting that dedicated cut-offs may be needed for different populations. On this note, the most recent AASLD guidelines have highlighted the lack of evidence to support the use of some NITs in primary care, raising concerns about underestimating liver disease, especially among diabetics [16].
Overall, there is increasing evidence suggesting that FIB-4, ELF and NAFLD fibrosis scores may be affected by the spectrum effect. Offering TE to high-risk patients in primary care could represent a way forward, as it is cost-effective and is not affected by spectrum biases [48]. Future work should focus on assessing the performance of NITs in true primary care cohorts and on the optimization of current referral management strategies.
This entry is adapted from the peer-reviewed paper 10.3390/metabo13040536
References
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023, 77, 1335–1347.
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321.
- Brunt, E.M.; Kleiner, D.E.; Carpenter, D.H.; Rinella, M.; Harrison, S.A.; Loomba, R.; Younossi, Z.; Neuschwander-Tetri, B.A.; Sanyal, A.J.; for the American Association for the Study of Liver Diseases NASH Task Force. NAFLD: Reporting Histologic Findings in Clinical Practice. Hepatology 2020, 73, 2028–2038.
- Glen, J.; Floros, L.; Day, C.; Pryke, R.; Guideline Development Group. Non-alcoholic fatty liver disease (NAFLD): Summary of NICE guidance. BMJ 2016, 354, i4428.
- Lembo, E.; Russo, M.F.; Verrastro, O.; Anello, D.; Angelini, G.; Iaconelli, A.; Guidone, C.; Stefanizzi, G.; Ciccoritti, L.; Greco, F.; et al. Prevalence and predictors of non-alcoholic steatohepatitis in subjects with morbid obesity and with or without type 2 diabetes. Diabetes Metab. 2022, 48, 101363.
- Younossi, Z.; Tacke, F.; Arrese, M.; Sharma, B.C.; Mostafa, I.; Bugianesi, E.; Wong, V.W.-S.; Yilmaz, Y.; George, J.; Fan, J.; et al. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology 2019, 69, 2672–2682.
- Estes, C.; Razavi, H.; Loomba, R.; Younossi, Z.; Sanyal, A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018, 67, 123–133.
- Younossi, Z.; Stepanova, M.; Ong, J.P.; Jacobson, I.M.; Bugianesi, E.; Duseja, A.; Eguchi, Y.; Wong, V.W.; Negro, F.; Yilmaz, Y.; et al. Nonalcoholic Steatohepatitis Is the Fastest Growing Cause of Hepatocellular Carcinoma in Liver Transplant Candidates. Clin. Gastroenterol. Hepatol. 2018, 17, 748–755.e3.
- Kleiner, D.E.; Makhlouf, H.R. Histology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis in Adults and Children. Clin. Liver Dis. 2016, 20, 293–312.
- Bugianesi, E.; Moscatiello, S.; Ciaravella, M.F.; Marchesini, G. Insulin resistance in nonalcoholic fatty liver disease. Curr. Pharm. Des. 2010, 16, 1941–1951.
- Neuschwander-Tetri, B.A. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: The central role of nontriglyceride fatty acid metabolites. Hepatology 2010, 52, 774–788.
- Guilherme, A.; Virbasius, J.V.; Puri, V.; Czech, M.P. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 2008, 9, 367–377.
- European Association for the Study of the Liver. Electronic address: ; Clinical Practice Guideline Panel; Chair; EASL Governing Board representative; Panel members. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis–2021 update. J. Hepatol. 2021, 75, 659–689.
- Barbosa, J.V.; Lai, M. Nonalcoholic Fatty Liver Disease Screening in Type 2 Diabetes Mellitus Patients in the Primary Care Setting. Hepatol. Commun. 2020, 5, 158–167.
- Kim, Y.; Chang, Y.; Cho, Y.K.; Ahn, J.; Shin, H.; Ryu, S. Obesity and Weight Gain Are Associated with Progression of Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2018, 17, 543–550.e2.
- Rinella, M.E.; Neuschwander-Tetri, B.A.; Siddiqui, M.S.; Abdelmalek, M.F.; Caldwell, S.; Barb, D.; Kleiner, D.E.; Loomba, R. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 2023. Publish Ah.
- National Guideline Centre (UK). Non-Alcoholic Fatty Liver Disease: Assessment and Management; National Institute for Health and Care Excellence (NICE): London, UK, 2016.
- Eslam, M.; Sarin, S.K.; Wong, V.W.S.; Fan, J.G.; Kawaguchi, T.; Ahn, S.H.; Zheng, M.H.; Shiha, G.; Yilmaz, Y.; Gani, R.; et al. The Asian Pacific Association for the Study of the Liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol. Int. 2020, 14, 889–919.
- Arab, J.P.; Dirchwolf, M.; Álvares-da-Silva, M.R.; Barrera, F.; Benítez, C.; Castellanos-Fernandez, M.; Castro-Narro, G.; Chavez-Tapia, N.; Chiodi, D.; Cotrim, H.; et al. Latin American Association for the study of the liver (ALEH) practice guidance for the diagnosis and treatment of non-alcoholic fatty liver disease. Ann. Hepatol. 2020, 19, 674–690.
- Ma, X.; Liu, S.; Zhang, J.; Dong, M.; Wang, Y.; Wang, M.; Xin, Y. Proportion of NAFLD patients with normal ALT value in overall NAFLD patients: A systematic review and meta-analysis. BMC Gastroenterol. 2020, 20, 10.
- Forlano, R.; Mullish, B.H.; Dhar, A.; Goldin, R.D.; Thursz, M.; Manousou, P. Liver function tests and metabolic-associated fatty liver disease: Changes in upper normal limits, does it really matter? World J. Hepatol. 2021, 13, 2104–2112.
- Davies, M.J.; D’Alessio, D.A.; Fradkin, J.; Kernan, W.N.; Mathieu, C.; Mingrone, G.; Rossing, P.; Tsapas, A.; Wexler, D.J.; Buse, J.B. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2018, 61, 2461–2498.
- Cusi, K.; Isaacs, S.; Barb, D.; Basu, R.; Caprio, S.; Garvey, W.T.; Kashyap, S.; Mechanick, J.I.; Mouzaki, M.; Nadolsky, K. American Association of Clinical Endocrinology Clinical Practice Guideline for the Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Primary Care and Endocrinology Clinical Settings: Co-Sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr. Pract. 2022, 28, 528–562.
- Bischoff, S.C.; Barazzoni, R.; Busetto, L.; Campmans-Kuijpers, M.; Cardinale, V.; Chermesh, I.; Eshraghian, A.; Kani, H.T.; Khannoussi, W.; Lacaze, L.; et al. European guideline on obesity care in patients with gastrointestinal and liver diseases–Joint ESPEN/UEG guideline. Clin. Nutr. 2022, 41, 2364–2405.
- Corey, K.E.; Kartoun, U.; Zheng, H.; Shaw, S.Y. Development and Validation of an Algorithm to Identify Nonalcoholic Fatty Liver Disease in the Electronic Medical Record. Dig. Dis. Sci. 2015, 61, 913–919.
- Rustgi, V.K.; Duff, S.B.; Elsaid, M.I. Cost-effectiveness and potential value of pharmaceutical treatment of nonalcoholic fatty liver disease. J. Med. Econ. 2022, 25, 347–355.
- Noureddin, M.; Jones, C.; Alkhouri, N.; Gomez, E.V.; Dieterich, D.T.; Rinella, M.E.; NASHNET. Screening for Nonalcoholic Fatty Liver Disease in Persons with Type 2 Diabetes in the United States Is Cost-effective: A Comprehensive Cost-Utility Analysis. Gastroenterology 2020, 159, 1985–1987.e4.
- Tanajewski, L.; Harris, R.; Harman, D.J.; Aithal, G.P.; Card, T.R.; Gkountouras, G.; Berdunov, V.; Guha, I.N.; Elliott, R.A. Economic evaluation of a community-based diagnostic pathway to stratify adults for non-alcoholic fatty liver disease: A Markov model informed by a feasibility study. BMJ Open 2017, 7, e015659.
- Vilar-Gomez, E.; Lou, Z.; Kong, N.; Vuppalanchi, R.; Imperiale, T.F.; Chalasani, N. Cost Effectiveness of Different Strategies for Detecting Cirrhosis in Patients With Nonalcoholic Fatty Liver Disease Based on United States Health Care System. Clin. Gastroenterol. Hepatol. 2020, 18, 2305–2314.e12.
- Congly, S.E.; Shaheen, A.A.; Swain, M.G. Modelling the cost effectiveness of non-alcoholic fatty liver disease risk stratification strategies in the community setting. PLoS ONE 2021, 16, e0251741.
- Johansen, P.; Howard, D.; Bishop, R.; Moreno, S.I.; Buchholtz, K. Systematic Literature Review and Critical Appraisal of Health Economic Models Used in Cost-Effectiveness Analyses in Non-Alcoholic Steatohepatitis: Potential for Improvements. Pharmacoeconomics 2020, 38, 485–497.
- Williams, R.; Aspinall, R.; Bellis, M.; Camps-Walsh, G.; Cramp, M.; Dhawan, A.; Ferguson, J.; Forton, D.; Foster, G.; Gilmore, I.; et al. Addressing liver disease in the UK: A blueprint for attaining excellence in health care and reducing premature mortality from lifestyle issues of excess consumption of alcohol, obesity, and viral hepatitis. Lancet 2014, 384, 1953–1997.
- Said, A.; Gagovic, V.; Malecki, K.; Givens, M.L.; Nieto, F.J. Primary care practitioners survey of non-alcoholic fatty liver disease. Ann. Hepatol. 2013, 12, 758–765.
- Standing, H.C.; Jarvis, H.; Orr, J.; Exley, C.; Hudson, M.; Kaner, E.; Hanratty, B. GPs’ experiences and perceptions of early detection of liver disease: A qualitative study in primary care. Br. J. Gen. Pract. 2018, 68, e743–e749.
- Islam, K.B.; Brandman, D.; Chu, J.N.; Goldman, M.L.; Fox, R.K. Primary Care Providers and Nonalcoholic Fatty Liver Disease: A Needs Assessment Survey. Dig. Dis. Sci. 2022, 68, 434–438.
- Spann, A.; Bishop, K.M.; Weitkamp, A.O.; Stenner, S.P.; Nelson, S.D.; Izzy, M. Clinical decision support automates care gap detection among primary care patients with nonalcoholic fatty liver disease. Hepatol. Commun. 2023, 7, e0035.
- Gracen, L.; Hayward, K.L.; Aikebuse, M.; Williams, S.; Russell, A.; O’Beirne, J.; Powell, E.E.; Valery, P.C. An exploration of barriers and facilitators to implementing a nonalcoholic fatty liver disease pathway for people with type 2 diabetes in primary care. Diabet. Med. 2022, 39, e14799.
- Anstee, Q.M.; Hallsworth, K.; Lynch, N.; Hauvespre, A.; Mansour, E.; Kozma, S.; Marino, J.P.; Bottomley, J.; Piercy, J.; Higgins, V. Real-world management of non-alcoholic steatohepatitis differs from clinical practice guideline recommendations and across regions. JHEP Rep. 2022, 4, 100411.
- Ratziu, V.; Anstee, Q.M.; Wong, V.W.; Schattenberg, J.M.; Bugianesi, E.; Augustin, S.; Gheorghe, L.; Zambon, V.; Reau, N. An international survey on patterns of practice in NAFLD and expectations for therapies-The POP-NEXT project. Hepatology 2022, 76, 1766–1777.
- Miller, M.H.; Fraser, A.; Leggett, G.; Macgilchrist, A.; Gibson, G.; Orr, J.; Forrest, E.H.; Dow, E.; Bartlett, W.; Weatherburn, C.; et al. Development and validation of diagnostic triage criteria for liver disease from a minimum data set enabling the ‘intelligent LFT’ pathway for the automated assessment of deranged liver enzymes. Front. Gastroenterol. 2018, 9, 175–182.
- A Usher-Smith, J.; Sharp, S.J.; Griffin, S.J. The spectrum effect in tests for risk prediction, screening, and diagnosis. BMJ 2016, 353, i3139.
- Vallet-Pichard, A.; Mallet, V.; Pol, S. FIB-4: A simple, inexpensive and accurate marker of fibrosis in HCV-infected patients. Hepatology 2006, 44, 769.
- Angulo, P.; Hui, J.M.; Marchesini, G.; Bugianesi, E.; George, J.; Farrell, G.C.; Enders, F.; Saksena, S.; Burt, A.D.; Bida, J.P.; et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007, 45, 846–854.
- Guha, I.N.; Parkes, J.; Roderick, P.; Chattopadhyay, D.; Cross, R.; Harris, S.; Kaye, P.; Burt, A.D.; Ryder, S.D.; Aithal, G.P.; et al. Noninvasive markers of fibrosis in nonalcoholic fatty liver disease: Validating the European Liver Fibrosis Panel and exploring simple markers. Hepatology 2008, 47, 455–460.
- Canivet, C.M.; Costentin, C.; Irvine, K.M.; Delamarre, A.; Lannes, A.; Sturm, N.; Oberti, F.; Patel, P.J.; Decaens, T.; Irles-Depé, M.; et al. Validation of the new 2021 EASL algorithm for the noninvasive diagnosis of advanced fibrosis in NAFLD. Hepatology 2023, 77, 920–930.
- Elangovan, H.; Rajagopaul, S.; Williams, S.M.; McKillen, B.; Britton, L.; McPhail, S.M.; Horsfall, L.U.; Valery, P.C.; Hayward, K.L.; Powell, E.E. Nonalcoholic Fatty Liver Disease: Interface Between Primary Care and Hepatology Clinics. Hepatol. Commun. 2020, 4, 518–526.
- Graupera, I.; Thiele, M.; Serra-Burriel, M.; Caballeria, L.; Roulot, D.; Wong, G.L.-H.; Fabrellas, N.; Guha, I.N.; Arslanow, A.; Expósito, C.; et al. Low Accuracy of FIB-4 and NAFLD Fibrosis Scores for Screening for Liver Fibrosis in the Population. Clin. Gastroenterol. Hepatol. 2021, 20, 2567–2576.e6.
- Forlano, R.; Jayawardana, S.; Mullish, B.; Yee, M.; Mossialos, E.; Goldin, R.; Petta, S.; Tsochatzis, E.; Thursz, M.; Manousou, P. Clinical and Cost-Effectiveness Analysis of Community-Based Screening Strategies for Non-Alcoholic Fatty Liver Disease in Patients with Type-2 Diabetes Mellitus. Available online: https://www.researchsquare.com/article/rs-2135338/v1 (accessed on 12 March 2023).
- Hinkson, A.; Lally, H.; Gibson, H.; Jones, R.; Rowe, I.A.; Shinkins, B.; Parker, R. Meta-analysis: Enhanced liver fibrosis test to identify hepatic fibrosis in chronic liver diseases. Aliment. Pharmacol. Ther. 2023, 57, 750–762.
This entry is offline, you can click here to edit this entry!
