You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Food Science & Technology
Carotenoids have important implications for human health and the food industry due to their antioxidant and functional properties. Their extraction is a crucial step for being able to concentrate them and potentially include them in food products. Traditionally, the extraction of carotenoids is performed using organic solvents that have toxicological effects. Developing greener solvents and techniques for extracting high-value compounds is one of the principles of green chemistry and a challenge for the food industry.
- green extractions
- green solvents
- carotenoids
- fruit by-products
- vegetable by-products
1. Introduction
The principles of green extraction dictate that it should be based on a process that reduces energy consumption, includes alternative solvents, uses renewable and natural products, and can guarantee a safe and high-quality extraction of bioactive compounds [34]. In traditional extraction and separation processes, huge quantities of organic solvents are used. Furthermore, the final extract may contain leftover solvents, impurities from the original material, or denatured chemicals as a result of the harsh extraction conditions [11]. In this way, an innovative process using green solvents and green techniques to avoid the degradation of the target compound is of great interest. There are several examples of green solvents, and they can be classified in diverse ways. Figure 1 shows the different solvents used for carotenoid extraction and their classification as green or not. Supercritical fluids (SCFs), vegetable oils, ionic liquids (ILs), natural deep eutectic solvents (NADESs), and limonene are some green solvents used for carotenoid extraction. Table 1 shows their advantages and disadvantages when used for green extraction compared with organic solvents. The characteristics, properties, optimal conditions, and most recent developments in the process used for the isolation of carotenoids from the green solvents in various different fruit and vegetable by-products are then explained and summarized in Table 2.
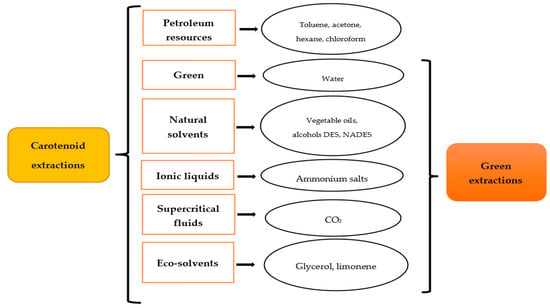
Figure 1. Alternative solvents for carotenoid green extraction (DES: deep eutectic solvents, NaDES: natural deep eutectic solvent).
Table 1. Advantages and disadvantages of green solvents for carotenoid extraction.
| Solvent | Advantages | Disadvantages | Environmental Impact | Cost |
|---|---|---|---|---|
| Supercritical fluids (e.g., CO2) |
Provide carotenoids with high purity. Little time needed. Non-explosive, nontoxic, and inexpensive technique. Avoids thermal degradation of carotenoids. |
High pressures needed. Limitations for samples containing large amounts of water. Require a large investment at the beginning. |
Low | Medium |
| Ionic liquids (e.g., ammonium salts) |
High biocompatibility with several compounds. Recyclability. Good chemical and electrochemical properties (volatility, stability, nonflammability, low melting point). |
High cost (5–20 times higher than conventional solvents). High viscosity. The preparation procedure involves the use of toxic compounds. |
Medium | Low |
| Vegetable oils (e.g., olive oil) |
Great solubility. Retards the oxidation time and degradation rate of the compounds. Eliminates the extra cost of evaporation. |
High viscosity producing low diffusivity and consequently low extraction yield. | Low | Low |
| Deep eutectic solvents and natural deep eutectic solvents (e.g., menthol) |
Their preparation is simple and cheap. Low toxicity and biodegradability. Recovery, recycling, and purification of the extracted compounds are easy. Wide range of combinations is estimated. |
High viscosity that produces problems with mass transfers and limits the extraction process. The application at an industrial scale might be difficult. |
Medium | Low |
| Terpenes (e.g., limonene) |
Consider GRAS. High biocompatibility with apolar compounds. Recyclability. |
Only can be used for lyophilic compounds. | Low | Medium |
Table 2. Carotenoid recovery using green solvents in fruit and vegetable by-products.
| Matrix | Solvent | Target Compounds | Yield | Extraction Method | Optimal Conditions | Isolation Process | Reference |
|---|---|---|---|---|---|---|---|
| Mango peel (Mangifera indica L.) |
Supercritical CO2 | All-trans- β-carotene | 6290 mg/100 g dry peel | SFE | 25.0 MPa, 60 °C and 15% w/w ethanol | Not necessary, directly include in food product | [35] |
| Mango peel (Mangifera indica L.) |
Supercritical CO2 | Total carotenoids | 560.4 mg/100 g dry peel | SFE | 30 MPa and 40 °C |
NA | [36] |
| Passion fruit bagasse (Passiflora edulis) |
Supercritical CO2 | Total carotenoids | 5.3 mg/100 g dry sample | SFE | 26 MPa and 60 °C |
NA | [37] |
| β -carotene | 1.6 mg/100 g dry sample | ||||||
| β-cryptoxanthin | 62.9 mg/100 g dry sample | ||||||
| Orange peel (C. sinensis L. Osbeck) |
Ionic liquid: 1-butyl-3-methylimidazolium chloride and ethanol (1:2) | Total carotenoids | 3.208 mg/100 g dry sample | UAE | 6 extraction repetitions of 5 min, 1:3 ratio | Resins (XAD-7HP) | [38] |
| Tomato wastes (Solanum lycopersicum) |
Ionic liquid: hexafluorophosphate 1-butyl- 3- methylimidazolium | Lycopene | 0.556 mg/100 g dry sample | Traditional extraction | NA | NA | [39] |
| Passion fruit peel (Passiflora edulis) |
Olive oil | Total carotenoids | 1.207 mg/100 g of dry sample | UAE | 39 min, 47 °C, 30/100 ratio | NA | [40] |
| Sunflower | 1.185 mg/100 g of dry sample | ||||||
| Pomegranate peels (Punica granatum L.) |
Sunflower oil | Total carotenoids | 0.613 mg/100 g of dry sample | UAE | 30 min, 51.5 °C, 0.10 g/mL ratio | Not necessary, directly include in food product | [41] |
| Mandarin epicarp (Citrus reticulata) |
Sunflower oil | β-carotene | 0.140 mg/100 g of dry sample | UAE | 60 min, 60 °C, 0.4 ratio | Not necessary, directly include in food product | [42] |
| Peach palm peels (Bactris gasipaes) |
Sunflower oil | Total carotenoids | 163.4 mg/100 g of dry sample | UAE | 30 min, 35 °C, and 1528 W/m2 | NA | [18] |
| Mango pulp (Chausa variety) |
Flaxseed oil | Total carotenoids | 0.841 mg/100 g of dry sample | High shear dispersion | 20,000 rpm, 4 min |
Not necessary, directly include in food product | [43] |
| Carrot wastes (Daucus carota) | Flaxseed oil | Total carotenoids | 77.48% of recovery | MAE | 9.39 min, 8.06:1 ratio, 165 W power | Not necessary, directly include in food product | [44] |
| Pumpkin peel (Cucurbita maxima) |
Corn oil | Total carotenoids | 3.803 mg/100 g of dry peel | UAE | 1:10 ratio, amplitude of 20%, 30 min, 22–25 °C |
Not necessary, directly include in food product | [45] |
| 3.494 mg/100 g of dry peel | MAE | ||||||
| Tomato wastes (Solanum lycopersicum) |
Sunflower oil | Lycopene | 91.4 mg/100 g dry sample | UAE | 70 W/m2 ultrasonic intensity and 10 min | Not necessary, directly include in food product | [46] |
| Carrot wastes (Daucus carota L.) |
Flaxseed oil | Total carotenoids | 3460 mg/100 g of dry sample | Magnetic stirrer | 10 min | Not necessary, directly include in food product | [8] |
| Buriti peel (Mauritia flexuosa L.) |
Choline chloride-based and ethanol | Total carotenoids | 1043 mg/100 g of dry sample | NA | 30 min, 50 °C, 0.1/2 ratio | NA | [47] |
| Apricot pulp (‘Bebekos’ cultivar) |
Choline chloride and L (+)-tartaric acid (2:1) and Methanol 80:20 (v/v) | β-carotene | 41.3 mg/100 g of dry sample | UAE | 10 min and 35 °C |
Not necessary, directly include in food product | [48] |
| 76.1 mg/100 g of dry sample | MAE | 20 min and 52 °C |
|||||
| Tomato by-products (Solanum lycopersicum) |
DL-menthol and lactic acid (8:1) | Lycopene | 1.446 mg/100 g of dry sample | UAE | 70 °C, 120 mL/g ratio, 10 min | NA | [49] |
| Pumpkin by-products (Cucurbita maxima) |
Caprylic acid: Capric acid (3:1) | β-carotene | 15,141 mg/100 g | UAE | 50 °C, 52.5 W/cm3 ultrasonic power, 7 mL/g ratio and 10 min | Switching NADES polarity | [50] |
| Orange peel (Citrus cinensis L. osbeck) |
D-limonene | β-carotene | 1125 mg/100 g of dry peel | UAE | 5 min, 20 °C, 1/10 ratio | Not necessary, directly include in food product | [13] |
SFE: supercritical fluid extraction, UAE: ultrasound-assisted extraction, MAE: microwave-assisted extraction, NA: not available.
2. Supercritical Fluids
One of the most important advantages of SCF extraction is allowing the extraction of bioactive compounds from plant material at low temperatures, which reduces the degradation of thermolabile compounds. Additionally, this process avoids the use of hazardous solvents, and also SCF is easy to remove from the target compounds [12]. Usually, SCF extraction uses CO2 because it possesses low critical constants (Tc = 31.1 °C, Pc = 7.38 MPa) and is denominated as a generally recognized as safe (GRAS) solvent, so it is safe for human consumption. Supercritical CO2 is a very nonpolar solvent, compatible for the extraction of low-polar compounds with small molecular weight such as triglycerides, fatty acids, aromas, and carotenoids [35,51]. Other fluids can be used in a supercritical state for the extraction of bioactives in fruit and vegetable materials, for example, ethane, propane, and dimethyl ether. These solvents have critical points comparable to CO2, and a higher polarity index, which makes them a better choice for the extraction of higher-polarity compounds [4,14,52].
SCFs have been recognized as successfully extracting carotenoids from different sources, such as the fruits, pulps, and wastes of passion fruit (Passiflora edulis), peach (Bactris gasipaes), apricot (Prunus armeniaca), banana (Musa X paradisiaca), etc. [36,51,53]. Sánchez-Camargo et al. [35] performed the extraction of carotenoids from mango (Mangifera indica L.) peel using CO2 as a SCF and ethanol as a co-solvent; the optimum conditions were 25.0 MPa, 60 °C, and 15% w/w ethanol with a result of 6290 mg all-trans-β-carotene/100 g dry peel. They demonstrated that the extracted all-trans-β-carotene can be included directly in sunflower oil to protect it against lipid oxidation without the solvent elimination process. These results can be compared with the experiment of García-Mendoza et al. [36], who also analyzed carotenoids in mango peels and obtained 560.4 mg/100 g of total carotenoids in dry peels at the optimum conditions of 30 MPa at 40 °C. Another example using CO2 as a SCF was performed by Filho et al. [54], who studied the carotenoids from the freeze-dried pulp waste of pitanga fruits (Eugenia uniflora L). The optimization showed the best conditions to be 60 °C and 25 MPa, and they succeeded in extracting 55% of the total carotenoid content, including 74% of the rubixanthin and 78% of the lycopene from the pulp. In the case of Viganó et al. [37], they extracted carotenoid from passion fruit by-products with optimum conditions (40 °C, 35 MPa) and achieved a recovery of 94.5% compared to the traditional Soxhlet extraction. They showed the effectiveness of SCFs for carotenoid extractions and how varying the conditions of pressure and temperature can extract different carotenoids. The high number of carotenoids extracted using CO2 could be related to molecular interactions between carotenoids and supercritical carbon dioxide, as well as its nonpolar character, which facilitates carotenoid diffusion from the vegetable membranes [37]. The use of supercritical CO2 constitutes an important extraction strategy due to its efficiency in the recovery of bioactive compounds. Additionally, target compounds can be obtained with no traces of solvent and can be directly included in the final food product. Additionally, it is environmentally friendly and nontoxic to human health, but it could require a large investment for industry at the beginning [23,55].
3. Ionic Liquids
ILs are organic salts showing melting points lower than 100 °C and have been successfully utilized for green extraction of carotenoids. Studies report that ILs assist with the permeabilization of the cell wall, interrupting the hydrogen bond network of cellulose [1]. One of the advantages of using ILs is their unique physicochemical properties, which depend on the structure. In addition, their insignificant volatility, nonflammability, and stability make them more attractive for bioactive compound extractions [17,56].
ILs can be used as a substitute for conventional organic solvents in the extraction process of a variety of compounds. However, carotenoid extraction using ILs has not been extensively studied. Murador et al. [21] developed a carotenoid extraction process from orange peels using ILs combined with ultrasound technique. They tested four different ionic liquids: 1-butyl-3-methylimidazolium chloride ([BMIM][Cl]), 1-n-butyl-3-methylimidazolium hexafluorophosphate ([BMIM][PF6]), 1-n-butyl-3-methylimidazolium tetrafluoroborate ([BMIM][BF4]), and 1-hexyl-3-methylimidazolium chloride ([HMIM][Cl]). Furthermore, the extraction yield was compared to that from a traditional extraction with acetone. It was demonstrated that the most effective IL was [BMIM][Cl], with a total carotenoid extract of 32.0 µg/g, while the result using acetone was 7.8 µg/g of dry matter. It is important to mention that the optimum conditions were a solid–liquid ratio of 1:3, an IL/co-solvent proportion of 1:2, and six extraction repetitions of 5 min. The effectiveness of ILs can be attributed to the affinity of [BMIM][Cl] with carotenoid compounds, hydrophobic and hydrogen bond interactions, and the aforementioned cellulose wall disruption activity of ILs, which can favor the solvent interaction with the target compound. In another study, the extraction of lycopene from tomatoes was performed using [BMIM][PF6] and [BMIM][Cl]. The results were in good agreement with the previous study, and the greater yields were extracted with [BMIM][Cl]. The total recovered was 5.560 µg/g of lycopene from tomatoes, compared to 3.650 µg/g with acetone, showing that IL application in the extraction process is a viable alternative [39]. Another study used the conductor-like screening model for real solvents (COSMO-RS) to predict the extraction efficiency of ILs, and it reported similar results. It demonstrated that imidazolium-based ILs exhibited higher cell disruption capability compared to pyridinium and ammonium-based ILs [7]. Extraction with ILs is an efficient option to extract carotenoid compounds, and selecting the optimum conditions of solid–liquid ratio, extraction time, use of co-solvents, and the best IL for a specific matrix are fundamental to favor the best interaction between the ILs and the target compounds.
The separation of biocompounds from the ILs has been reviewed, and the extraction, purification, and recovery of several compounds (caffeine, hydrolyzable tannins, and gallic acid), as well as the recycling and reuse of ILs, has been considered [15]. However, in the literature there are few studies that describe integrated and full processes for the isolation and recovery of carotenoids from ILs. Back-extraction techniques and antisolvent precipitation of bioactives are the most commonly used methods. The use of ion-exchange resins, microporous resins, distillable ILs, and thermoresponsive polymeric ILs are some other alternatives. One example of a recovery process for orange peel carotenoids from ILs was developed by Murador et al. [21]. They used XAD-7HP resin with a recovery of the ILs from 59.5–63.8% and carotenoids from 52.2–58.7%, which raises the possibility of including the obtained extracts directly in food products as a natural pigment. Nevertheless, to achieve better results and determine IL effectiveness, more research is needed to optimize the recovery process.
4. Vegetable Oils
Carotenoids are oil-soluble pigments and the use of oils as solvents can be an excellent alternative to replace organic solvents. The use of vegetable oils is according to the principles of a green process, because they are environmentally friendly solvents and produce an extract without contaminants. One of the most important advantages of the use of oils for carotenoid extraction is that elimination of the solvent is not necessary, and the extracted compound can be included directly in final food products [40]. Additionally, oils play a role as an impediment against oxygen, retarding the oxidation time and degradation of the carotenoid extracts [41].
There are some studies showing the use of vegetable oils as an alternative for carotenoid extraction. The most popular oils used are flaxseed, olive, soy, sunflower, corn, and peanut. Elik et al. [44] studied the extraction of carotenoids from carrot juice wastes using flaxseed oils in microwave-assisted extraction, and they recovered 77.48% of carotenoids at the optimal conditions of 165 W, 9.39 min, and a 8.06:1 g/g oil-to-waste ratio. They also compared this result to the traditional extraction using n-hexane, ethanol, and acetone (2:1:1 v/v/v), where the recovery was about 87% of the carotenoids in 120 min. Traditional extraction had a higher extraction yield, but the elimination of the organic solvent takes a long time and is energy-consuming. It is important to mention that, in the previous case, the result was an enriched oil with carotenoids, phenolics, and antioxidants.
There is also the case of Goula et al. [41], who extracted carotenoids from pomegranate peels with sunflower oil and soy oil. Their results demonstrated that combining the use of green solvents with ultrasound successfully extracted 0.620 and 0.670 mg carotenoids/100 g of dry peels using sunflower oil and soy oil, respectively. Sharma and Bhat [45] compared the extraction of carotenoids in pumpkin peels using corn oil with that using hexane/isopropyl alcohol as a traditional solvent. The results showed that almost twice as much total carotenoid was extracted when employing a green solvent (33.70 µg/g) compared to that using conventional extraction (16.20 µg/g). One aspect in the selection of the right oil is the viscosity; a low viscosity is associated with a better migration through the matrix, and consequently, the extraction yield increases [57]. The extraction efficiency of carotenoids using oils can also depend on other factors including polarity, the amount of phospholipids, and chain length of fatty acids [58]. Oils with higher quantities of short-chain fatty acids and lower amounts of phospholipids can improve carotenoid extraction [59].
The selection of the optimal conditions for carotenoid extraction using vegetable oils is an important step, due to their easy degradation under exposure to light, heat, and oxygen for a long term. For this reason, some studies have optimized the extraction process and considered variables such as intensity or power employed, time, temperature, and solid–liquid ratio [60]. Ordoñez et al. [18] performed the extraction of carotenoids from peach palm fruit using ultrasound-assisted extraction and sunflower oil as a solvent. The optimal ultrasound-assisted extraction (UAE) conditions were obtained with an ultrasonic intensity of 1528 W/m2, extraction temperature of 35 °C, and extraction time of 30 min. Under those conditions, carotenoid recovery was 163 mg/100 g of dry peel. Chutia and Mahanta [40] found similar trends using ultrasound and olive oil in passion fruit peels. The optimum conditions were 39 min treatment time, 47 °C temperature, and 30 g/100 mL solid–liquid ratio. It was shown that the extraction yields of carotenoids are time-dependent, and yields increased with the ultrasound time increasing from 10 to 40 min. Temperature is another important variable to consider in the optimization process. In these studies, it was shown that increasing the temperature to around 50 °C, but no higher than 60 °C, increases the extraction yield. Additionally, a higher temperature decreases oil viscosity; so, consequently, a greater fluidity facilitates the diffusion of extractable lipophilic compounds [18]. These results show that the use of vegetable oils could efficiently replace organic solvents because they can extract the same amount or even more than conventional solvents, and furthermore, they can be included in the final food products, avoiding the process of solvent elimination.
5. Deep Eutectic Solvents and Natural Deep Eutectic Solvents
Deep eutectic solvents (DESs) are fully compliant with all 12 principles of green chemistry; they are nontoxic, inexpensive, biodegradable, and environmentally friendly [23,34]. Additionally, they have gained interest in recent years due to their cost–benefit balance and easy preparation, since the cost of DESs is comparable to that of conventional solvents [12,61]. When natural components are used for the preparation of the eutectic mixture, they are considered natural deep eutectic solvents (NADESs). DESs and NADESs are mixtures of two or three components forming hydrogen bonds. The hydrogen bond acceptor is an organic salt (quaternary ammonium or phosphonium salt) and the hydrogen bond donors are sugar, alcohol, amino acid, organic acid, etc. [62]. These solvents are considered an interesting alternative because of their recycling potential and easy purification of the extracted bioactive compounds. The main drawback of these solvents is their high viscosity, which produces mass transfer problems and restricts the extraction process [63,64]. Studies recommend reducing the viscosity by adding water in the range of 5–30%, but this process can limit their capacity for extracting hydrophobic compounds [62,65]. DESs are also considered promising solvents for green extraction processes due to the formation of strong hydrogen bonds between their components and the extracted compounds, and high quantities of water can decrease this interaction [66,67].
The use of choline chloride-based deep eutectic solvents as a co-solvent for the extraction of carotenoids in Buriti fruit (Mauritia flexuosa) wastes was studied, and the recovery was 10.4 mg/g. It was demonstrated that it did not increase the ethanolic extraction yield because β-carotene, which is the carotenoid usually present in fruits and vegetables, does not have the functional group for interacting with the hydrophilic choline chloride-based DES [47]. Additionally, it is well-known that all DESs with concentrations ranging from 50–30% of water presents low carotenoid extraction yields. Another parameter that must be considered in DES extractions and optimization processes is viscosity. A higher solvent viscosity limits the solute migration from the solid matrix to the liquid medium and, in consequence, a lower yield is extracted [68]. In another study, with a different combination of components, choline chloride and tartaric acid in apricots, it was reported that these solvents extracted 41.30 mg/100 g of the dry sample while organic solvents extracted 11.50 mg/100 g [48]. In that study, ethanol was used as a co-solvent to decrease the viscosity and recover a higher yield of carotenoids.
The most characterized DESs are water-soluble; thus, their application to hydrophobic compounds is limited and there are few studies using DESs for carotenoid extraction [69]. Recently, the use of hydrophobic DESs has been studied to extract nonpolar phytochemicals [70]. The first publication about hydrophobic DESs involved using a fatty acid (decanoid acid) as a hydrogen-bond acceptor (HBD) and a quaternary ammonium salt as a hydrogen-bond donor (HBA) to extract volatile fatty acids (VFAs) [71]. However, their use to extract carotenoids from fruit and vegetable by-products is still narrow. Silva et al. [49] performed the extraction of lycopene from tomato waste using a hydrophobic eutectic mixture (HEM) compound of DL-menthol as HBA and lactic acid as HBD, and UAE was used. The optimum conditions were 70 °C, 8:1 mol HBA/mol HBD, 120 mL/g solvent: sample, and 10 min of extraction time. The results showed an excellent capacity for extracting lycopene, with a yield of 1446 mg/g. Stupar et al. [50] extracted β-carotene from pumpkin by-products using hydrophobic NADESs based on fatty acids. Caprylic acid: capric acid (3:1) was selected as the optimal NADES, obtaining 151.41 µg/mL yield of β-carotene at the optimum conditions of 50 °C, ultrasonic power of 60% (52.5 W/cm3), and a solvent-to-solid ratio of 7 mL/g during 10 min of extraction.
The excellent extraction capacity of DESs and their low or nonexistent toxicity allow their employment in food without further isolation. However, an efficient method for separation and DES reusability must be developed before DESs can be employed in industrial applications as a substitute for conventional organic solvents [72]. Stupar et al. [50] proposed a sustainable method for the separation of carotenoids from hydrophobic NADESs. This consists of switching solvent polarity from hydrophobic to hydrophilic adding water and ammonium hydroxide. The pH of the solvents was easily changed, and as a result the polarity of the NADES extract also changed. They obtained precipitated carotenoids because of their low solubility in the hydrophilic media. After the separation of carotenoids, the solvent could be reused in hydrophilic form or switched back to hydrophobic using CO2.
The use of DESs as an innovative green alternative to the traditional carotenoid extraction process for the revalorization of food wastes and by-products is increasing, but the number of studies is still limited. The variables, optimum conditions, and characteristics of the DESs could change for each matrix and more studies in this area are needed. Furthermore, their potential inclusion in food products directly is a promising alternative for industry, but the development of databases containing information on their physicochemical qualities and toxicity is needed.
6. Limonene
Limonene is a cyclic monoterpene and is one of the most important essential oils in citrus fruit skins. There are two isomers, D- and L-limonene, and D-limonene represents 90% of total citrus essential oils. D-limonene is recognized for its antimicrobial and antioxidant properties and can be exploited as an antioxidant agent in the food industry [73]. Limonene does not show any functional groups available for hydrolysis; therefore, it seems to be a highly lipophilic solvent [74]. Recently, due to its highly nonpolar properties, researchers have found this compound is highly suited to replace petroleum-based solvents.
The evidence for the use of D-limonene for carotenoid extractions is still limited, but the effectiveness of this compound as a solvent has been demonstrated. Boukroufa et al. [13] used D-limonene to extract carotenoids from orange peels and compared it with a traditional extraction using hexane. They obtained yields of 11.25 mg/ L at the optimal conditions of 208 W/cm−2, 20 °C, and 5 min of extraction. They observed that the carotenoid content was practically the same for both solvents, which indicates that limonene can be used as a replacement for traditional organic solvents. Another important point in that study was regarding the recycling of the solvents used, and it was demonstrated that hexane allows the recovery of only 50% of the solvent against 90% for D-limonene. Moreover, the extracts obtained using D-limonene can be directly used in food products because it they are GRAS by the US Food and Drug Administration.
This entry is adapted from the peer-reviewed paper 10.3390/foods12040863
This entry is offline, you can click here to edit this entry!
