Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Neurosciences
NREM sleep parasomnias mainly include confusional arousals (CA), sleep terrors (ST), and sleepwalking (SW), three clinical entities lumped together under the name of disorders of arousal (DoA).
- NREM sleep parasomnias
- disorders of arousal
- sleepwalking
- confusional arousal
1. Clinical Interview
An accurate clinical interview is theoretically sufficient to confirm a diagnosis of DoA according to standard international criteria [1,10] (see Table 1). However, limiting the diagnosis to clinical criteria may present limitations related to the age of occurrence of the episodes and the possibility to collect an accurate description of the episodes from the patients and their relatives/bed partners.
The presence of recurrent incomplete awakenings (criteria A, ICSD-3) associated with an inappropriate or absent responsiveness to the external environment (criteria B in ICSD-3) represents the very core of the definition of DoA [1]. During CA, patients may sit up in bed, stare straight ahead or look around the room with a perplexed or blunt expression, may vocalize intelligible or unintelligible words, and may often return back to sleep soon after. ST usually begins abruptly, with an intense autonomic activation (tachycardia, tachypnea, diaphoresis, mydriasis). Patients may rise screaming and appear scared and unresponsive to external attempts to interact or calm them down. During SW, patients leap out of bed and wander about the room or the house, or more rarely, may even walk outside. SW patients may perform routine activities like dressing, washing, preparing for school, urinating (sometimes at inappropriate locations), but can also engage in more dangerous actions like moving the furniture, manipulating sharp objects, or jumping out a window [1,11,12,13,14,15]. Prolonged cases associated with sleep driving have been described [16]. Notably, the arousal threshold is higher than wakefulness, and pain processing seems to be altered [17], further exposing these patients to potential dangers.
The ICSD-3 recognizes some other common features in DoA, meaning a limited or absent cognition or dream imagery during the episodes (criteria C in ICSD-3 and criteria B in DSM-5) and partial-to-complete amnesia for the episodes (criteria D in ICSD-3 and C in DSM-5) [1]. Frequent amnesia significantly undermines history taking and makes critical the presence of caregivers or bedpartners who witnessed the episodes during the clinical interview. Interestingly, the view of DoA as automatic behaviors performed in the absence of any conscious experience has been significantly challenged in the last two decades. In fact, different retrospective studies have shown various degrees of mental/oneiric recall associated with DoA episodes (above 70% of patients across their life span) [18,19,20]. Mental experiences during DoA episodes seem not only limited to single visual scenes or simple mental fragments, and even complex, vivid and long dream-like/hallucinatory experiences may not rule out a diagnosis of DoA [21]. In the only case series where patients with ST were interviewed immediately after their episodes, mental imagery was present in ~ 60% of the cases [22]. The degree of recall may depend on several factors, including the time distance between the clinical interview and the episode’s occurrence, as well as the individual mnesic ability (usually more developed in adults). Of note, the recall rate seems to differ between children and adults, in line with the hypotheses of more immature sleep mechanisms in children (and lower dream recall rate), and with an easier arousability in adults (that may facilitate the incorporation of the dream experience in long-term memory) [5,20]. Thus, we suggest that criteria C and D should be considered optional supportive criteria (especially in children) but not mandatory criteria (see Table 2). Of note, in adults, mental content may also have a discriminatory value against RBD. Indeed, while negative emotions and violent behaviors are as frequent in DoA as in RBD [19], high levels of apprehension associated with misfortune (meaning any threat over which the subject does not have control) is more strictly associated with DoA than RBD. Self-defense or fleeing behaviors are more typical of DoA and direct fighting/aggressive behaviors (where the patient acts the first move) or aggressions by either humans or animals are more frequent in RBD [18,19]. Moreover, the occurrence in at-home settings (and not in a virtual dreaming scenario as in RBD) seems to be very specific for DoA [23].
Table 2. Suggestions for new DoA diagnostic criteria.
| Definition | |
|---|---|
| Clinical Manifestation |
|
| Distress/Disability |
|
| Exclusion |
|
| Levels of certainty |
|
CA, ST, and SW should be interpreted as a diagnostic continuum on the basis of clinical, epidemiological, physiological, and genetic evidence [2]. Indeed, patients usually display more than one clinical subtype (CA, ST or SW) throughout their lifespan and may even switch from one manifestation to the other within the same episode. Of note, the presence in the same subject of different semiological manifestations across episodes and a lack of stereotypy within the episodes, together with partial interactions with the surroundings (e.g., environment exploration and apparently purposeful object manipulation) seem to be specific for DoA. These elements support the differential diagnosis with sleep-related hyper-motor epilepsy (SHE)—the condition that poses more challenging issues in terms of differential diagnosis, especially in the presence of short episodes [11,24,25]. Contrary to REM sleep behavior disorder (RBD), eyes during DoA episodes are wide open [26], even during minor episodes, and movements more often involve the trunk [27].
The timing and the evolution of episodes are other essential features to consider. Indeed, DoA episodes more frequently—although not exclusively—occur during the first third of the night (when slow wave sleep (SWS) predominates), while RBD more typically occurs in the second half of the night (when REM sleep predominates); SHE tends to manifest more equally across the entire night [28]. Last but not least, DoA episodes usually occur in clusters across the life span, with a frequency of episodes that fluctuates over time, possibly in relation to sleep deprivation and stress [12].
Another fundamental part in DoA clinical history is the identification of predisposing, priming, and precipitating factors like the familiar history [29], the presence of symptoms or signs suggestive for other sleep disorders known to fragment sleep (like sleep apneas or periodic limb movements), an unusual sleep environment, sleep deprivation, sensory stimuli, or medications [30,31].
2. Questionnaires
Few questionnaires have been validated for screening patients with a clinical suspect of NREM sleep parasomnias and/or quantify their severity. These scales have been validated mainly in adult populations.
The Munich Parasomnia Screening (MUPS) questionnaire [32] is a self-rating instrument composed of 21 items and provides a quick overview on the occurrence and frequency of parasomnias and other nocturnal behaviors in adults. However, the protocol of validation did not include a video-polysomnography (VPSG) to confirm the diagnosis, as this questionnaire was meant more for screening than for diagnostic purposes [32,33].
The Paris Arousal Disorders Severity Scale (PADSS) [34] is a useful instrument for monitoring DoA clinical symptoms and severity. It is composed by a self-rated scale assessing the occurrence of 17 parasomnia behaviors, their frequency, and consequences. Of note, neither MUPS or PADSS were validated against patients with SHE [35].
To this extent, the Frontal Lobe Epilepsy and Parasomnias (FLEP) Scale was developed to differentiate SHE from parasomnias, especially DoA [36]. However, not all the patients in this study had a diagnosis confirmed by VPSG. A successive Italian validation of the same scale [37], in which the diagnoses were confirmed by in-lab VPSG, did not confirm these encouraging results. The scale gave an incorrect diagnosis in 5.6% of cases, which included patients with SHE seizures characterized by nocturnal wandering (mistaken for SW), while around one-third of cases had uncertain diagnostic indications, due to items associated with a risk of misdiagnosis (mainly “recall” and “clustering” of the events, increasing the chance of mistaking RBD for seizures) [37].
The Arousal Disorders Questionnaire (ADQ) was recently validated to screen adults with DoA according to ICSD-3 criteria [38]. The questionnaire includes two parts: the first part is specific for each subtype (CA, ST, SW), while the second part assesses general DoA criteria. This questionnaire has a good interobserver reliability [39], an acceptable sensitivity and a high specificity for the diagnosis of DoA. Excluding the items regarding consciousness and episode recall (ICSD-3 criteria C and D, see Table 3), the sensitivity increased without major changes in specificity, highlighting that these criteria might not be always appropriate for adult cases of DoA.
Overall, the use of validated questionnaires might support the screening of DoA in non-specialized settings, improve DoA diagnostic accuracy in clinical and research sleep settings, assess the severity of DoA over time, before and after treatment, and guide further diagnostic assessments in case of atypical scenarios or comorbidities.
Table 3. Scales available for NREM sleep parasomnias screening, diagnosis, or follow-up.
| Validation Sample | Sensitivity | Specificity | Utility | |
|---|---|---|---|---|
| MUPS [32] | 65 adult psychiatric patients 50 adult patients with sleep disorders 65 adult HC |
ST: 100% CA: 100% SW: 83% |
ST: 89% CA: 97% SW: 100% |
Screening of parasomnias, sleep-related movement disorders or normal variants |
| PADSS [34] | 73 adult and adolescent active SW/ST 45 adult and adolescent patients with sleep disorders (26 former DoA, 19 RBD) 53 adult and adolescent HC |
DoA: 84% | DoA: 88% | DoA severity and follow-up |
| FLEP [36] | 62 adult and children/adolescent patients (31 with SHE, 29 with DoA, 2 with RBD) | SHE: 100% | SHE: 90% | Differentiate SHE from parasomnias |
| FLEP [37] | 71 adult patients (11 DoA, 14 SHE and 46 RBD) | SHE: 71% | SHE: 100% | Differentiate SHE from parasomnias |
| ADQ [38] | 47 adult and adolescent DoA 103 adult and adolescent patients with sleep disorders (56 RBD, 39 SHE, 6 NES, 2 with drug-induced DoA) |
DoA: 72% (83% without criteria C and D) |
DoA: 96% (93% without criteria C and D) |
DoA diagnosis |
DoA, Disorders of Arousal; RBD, REM behavior Disorder; SHE, Sleep-related hypermotor epilepsy; NES, Night Eating Syndrome; ST, Sleep Terror; CA, Confusional Arousal; SW, Sleepwalking; HC, healthy control subjects.
3. Video-Polysomnography
VPSG is usually performed when evaluating patients with atypical NREM sleep parasomnias, meaning cases with red flags, such as (1) adult onset; (2) stereotypical, repetitive, abnormal unnatural postures, ballistic movements or focal motor patterns; (3) high episode frequency; (4) episodes of brief duration; (5) episodes recurring in any part of the night; (6) other suspected sleep disorders; and (7) presence of dissociative symptoms [40] during daytime/other major psychiatric comorbidities [41] (see Figure 1).
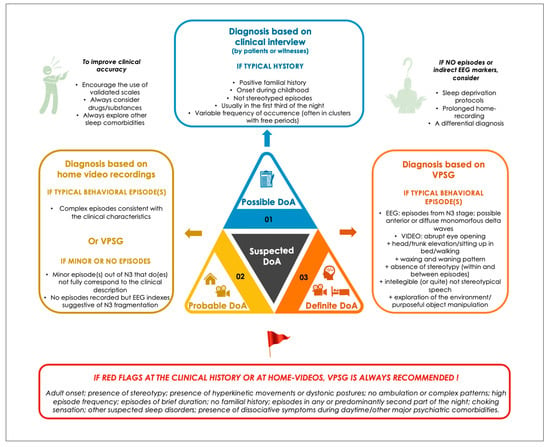
Figure 1. Proposed levels of certainty in DoA diagnosis.
VPSG assumes an important role in the differential diagnosis between DoA and SHE [35], RBD or parasomnia overlap disorder [42], as well as in sleep-related (psychogenic) dissociative disorders [43]. Furthermore, VPSG traces can give important information regarding other potential sleep disorders triggering DoA, such as obstructive sleep apneas and periodic limb movements (PLM) (up to 33% of the cases in adults) [44].
Moreover, in the assessment of DoA, video synchronized with EEG adds critical information. The probability of catching a typical episode during VPSG is approximately 30–60% [44,45,46,47]. This percentage largely depends on the frequency/severity of parasomnia episodes, the life period when the recording is performed, the patient’s age, and the sensibility of the scorer to minor/brief nocturnal episodes [44,45,46,47], which represent the majority of episodes captured in-laboratory.
4. Home-Video-Recordings
Despite being the current gold standard for the assessment of complex nocturnal sleep behavior, supervised VPSG is expensive, time consuming, and often implies long waiting lists. Furthermore, as highlighted in the previous section, the probability of catching at least one parasomnia episode during a single-night VPSG assessment is far from being satisfactory. This percentage largely depends on the frequency/severity of parasomnia episodes, the patient’s age, and the sensibility of the scorer to minor/brief nocturnal episodes [44,45,46,47], which represent the majority of episodes captured in-laboratory. Indeed, the differential diagnosis of brief nocturnal episodes by both video and EEG is often extremely difficult [11]. In this perspective, the analysis of homemade video recordings of nocturnal episodes may be an important supportive diagnostic tool for DoA [78]. It offers multiple advantages: wide availability, low-costs, the possibility of recording patients in their usual sleep environment and of repeated recordings, increasing the chances of capturing multiple and possibly longer and more complex episodes compared to the standard in-laboratory setting.
Even in the case of partial recordings by parents/bed partners, when the onset of the episode is missed, video-recordings could be a useful tool for the differential diagnosis of nocturnal events, as it is not the onset of the episodes but the evolution and the offset of the events that has a discrimination value between DoA and SHE [78]. Furthermore, repeated home videos using professional high-quality infra-red motion-detector cameras can now record and store consecutive episodes from several nights.
Up to now, 2 single case reports highlighted the usefulness of monitoring nocturnal behaviors in adults with NREM sleep parasomnias with an infrared camera in the home environment for 5 and 2 weeks, respectively [21,79]. More recently, a study of 20 adults with frequent DoA episodes, recorded for at least 5 consecutive nights, confirmed a good feasibility, acceptability, and clinical value of home-video recordings in DoA [80]. An average of at least 3 nights was required to capture at least 1 event and events were usually more complex than those recorded during VPSG.
5. Differential Diagnoses
DoA must be differentiated by a number of other sleep disorders [81] (see Table 4): REM-related parasomnias, like nightmare disorder, RBD, and parasomnia overlap disorder [82]. SHE and sleep related psychogenic dissociative disorder (SRDD) are probably the trickiest conditions that might simulate DoA [40,43], in the absence of objective findings. Nocturnal panic attacks may also simulate ST [83,84,85]. Medication- or substance-induced nocturnal confusion must be always ruled out before considering a DoA diagnosis.
Table 4. DoA differential diagnoses.
| DoA | RBD | SHE | SRDD | Nightmare Disorder | Nocturnal Panic Attacks | |
|---|---|---|---|---|---|---|
| Age at onset | Infancy | >50 years, rare in children/adolescent (mostly Narcolepsy patients) | Any age | Variable | Children>adults | Variable—adults |
| Gender | M = F | M in sleep clinics, equal in general population | M > F | F > M | M = F | F in DP/NP, M in isolated NP |
| Predisposing or priming conditions |
Family history, stressful situations, sleep deprivation | - | - | Traumatic events/abuse/major psychopathology | Traumatic events | Mostly DP attacks |
| Triggers | + | - | +/− | + | + | + |
| Occurrence during the night | Usually, first third | Second part of the night | Any time | W close to bedtime | Second part of the night | First third |
| Sleep stage | N3 | REM sleep | N1, N2 | Prolonged W | REM sleep | Transition N2–N3 |
| Frequency (number of episodes/ night) |
One major, possible multiple minor | Variable, from one to several | Several | From one to several | Usually, one | Usually one, occasionally > one |
| Episodes duration |
1–10 min | Variable, seconds to several minutes | Seconds to 3 min | >1 h | 3–30 min | 2–8 min |
| Stereotypy | - | - | + | - | - | - |
| Mental content |
Short scenes involving misfortune or threat | Complex scenarios, variable content from aggression to positive | - | - | Nightmare, intense fear | - |
| Consciousness at the end of the episodes |
Impaired | Preserved | Variable | Preserved | Preserved | Preserved |
| Recall | Rare in children, > in adults | Frequent | Inconstant | Inconstant | Present | Vivid recall of fearful sensations, not related to dream scenario |
| Clinical consequences |
Sleepiness, injuries, daytime impairment | Injuries | Injuries, daytime impairment | Self-inflicted injuries | Mood disturbance, daytime impairment, bedtime anxiety | Daytime disfunction |
M, males; F, females; DoA: disorders of arousal; DP, diurnal panic; NP, nocturnal panic; PTSD, post-traumatic stress disorder; SHE: sleep-related hypermotor epilepsy; RBD: REM-sleep behavior disorder; SRDD: sleep-related dissociative disorder; W, wake; N1: sleep stage 1, N2: sleep stage 2; N3: sleep stage 3.
6. Therapy
Current evidence on both pharmacological and non-pharmacological interventions in NREM sleep parasomnias is hampered by: (1) the relative low number of patients that reach specialistic attention; (2) the lack of agreement between specialized sleep centers; (3) the lack of animal models and of a clear understanding of neural circuits implied in NREM sleep parasomnias; and (4) the fact that treatment efficacy is particularly insidious to test, given the frequent episode-related amnesia and the consequent relatively low reliability of subjective reports. Only one study using an objective outcome (number of episodes captured with home-video recordings) has been conducted to date. This study suggested a reduction in the frequency of episodes in eight DoA patients treated with Clonazepam [80]. Treatment strategies currently available for DoA has been extensively reviewed elsewhere [86,87,88,89,90]. We will briefly summarize them below and in Table 5.
Table 5. Treatment approaches available for DoA.
| Non-Specific Interventions | |||
|---|---|---|---|
| Reassurance | |||
| Parents/bedpartners education | |||
| Sleep hygiene | |||
| Environmental safety | |||
| Treatment of other sleep disorders | |||
| Removal of triggering medications | |||
| Pharmacological interventions | |||
| Age group | Efficacy | Type of evidence | |
| Clonazepam | Adults | >70% | Large case series Case reports/small case series |
| Other benzodiazepines | Adults | Insufficient/ contradictory results |
Case reports/small case series |
| Antidepressants | Adults | Insufficient/ contradictory results |
Case reports/small case series |
| 5-L-OH-Tryptophane | Children | >80% | Open clinical trial (ST only) |
| Melatonin | Adults - Children |
Insufficient/ contradictory results |
Small retrospective case series/case reports |
| Non-pharmacological interventions | |||
| Age group | Efficacy | Type of evidence | |
| Scheduled awakenings | Children | Insufficient results | Small case series |
| Hypnosis | Adults - Children |
Positive results | Rater-blind trial/Case series |
| Integrated CBT protocols | Adults | Positive results | Retrospective case series Randomized control trial |
| Other specific non-pharmacological approaches | Adults - Children |
Insufficient results | Case reports/Case series |
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics13071261
This entry is offline, you can click here to edit this entry!
