NCT could be helpful in a day-to-day clinical setting that involves dealing mostly with normal patients undergoing routine checkups. This type of tonometry can be ideal as a screening tool, which can easily be performed by non-medical staff. Although studies have shown that NCT tends to overestimate GAT measurements, NCT can prove to be useful for post-operative patients with lid edema, limited collaboration, ocular pain, discomfort and increased tear film meniscus size, which are all factors that influence proper GAT measurements. NCT can be a useful screening tool, but should never replace or be interchanged with GAT, especially in the management of patients with risk factors, ocular hypertension, suspect patients and glaucoma.
This instrument provides IOP measurements based on the indentation principle, in addition to pachymetry taken by on optical device and other biomechanical parameters of the cornea obtained by registering the surface deformation due to an applied air pulse, similar to an ORA device. A Scheimpflug camera visualizes an 8.5 mm diameter of the center of the surface of the cornea and precisely records the corneal deformation induced by the air-jet and its return to its normal shape with a high resolution and more than 4300 frames per second. A biomechanically corrected IOP value (bIOP), which takes the individual corneal deformation parameters into account, is also provided by the device.
The Corvis ST precision for the CCT and IOP values has been shown to be excellent; however, it is moderate for the corneal deformation parameters [
62,
63,
64]. Previous studies demonstrated that Corvis ST tends to underestimate IOP readings obtained with GAT [
61,
62,
65,
66]. The Corvis ST biomechanically corrected IOP values (bIOP) have been shown to be less influenced by the CCT and corneal biomechanics and to be more effective in measuring the IOP in subjects who underwent refractive surgery [
67]. Moreover, the Corvis ST corneal deformation parameters have been shown to be effective in discriminating between normal and keratoconic eyes [
68].
5. Pneumotonometry
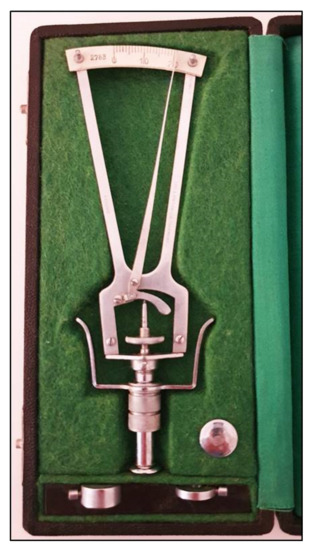
Pneumotonometers are devices based on the applanation principle, which use a different technology [
69,
70]: the tonometer probe consists of a hollow central tube flanked by a side exhaust, and the sensor is air pressure, which is dependent on the resistance of the exhaust. During the cornea applanation, the pressure within the central tubes increases to match the force generated by the IOP. A pneumatic electronic transducer converts the air pressure to a tracer on a strip of paper (
Figure 9).
Figure 9. Pneumotonometer.
In several studies, pneumotonometry proved to be quite accurate and reliable in glaucoma screening and showed a greater reliability compared to GAT after PRK and LASIK [
71,
72,
73]. Pneumotonometers such as the Pulsatile Ocular Blood Flow (OBF,
Figure 10) have been used in the past to measure the pulse fluctuation and thereby give indirect information regarding the ocular blood pulse [
74,
75,
76,
77]. OBF measurements, however, appear to be more influenced by CCT and more variable than GAT readings, with a significant overestimation [
78,
79,
80]. The clinical usefulness of this instrument in clinics still remains controversial.
Figure 10. Langham Ocular Blood Flow pneumotonometer.
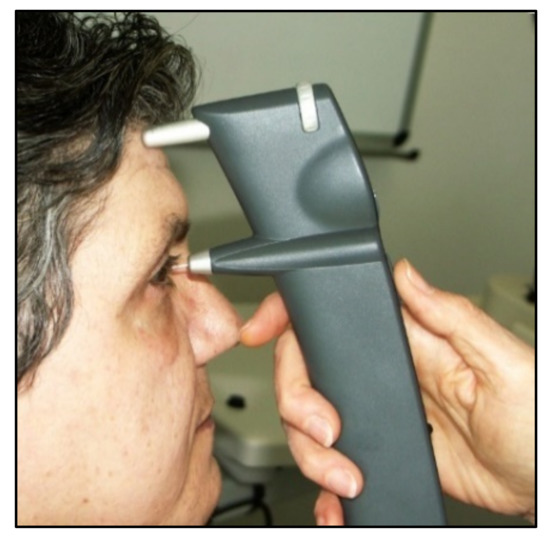
6. Rebound Tonometry
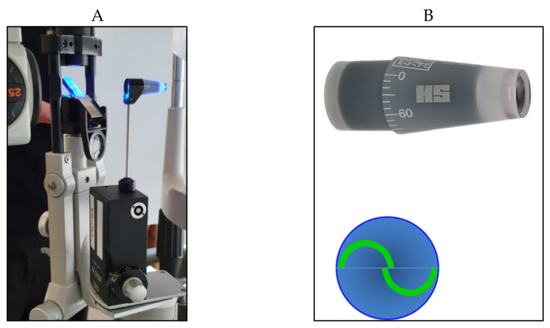
From a clinical point of view, the iCare rebound tonometer, introduced in 2000 by Kontiola [
81], is currently one of the most interesting and widespread instruments used in practice (
Figure 11).
Figure 11. iCare rebound tonometer.
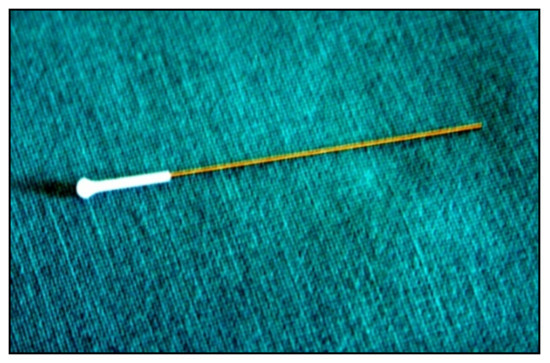
A subtle probe impacts onto the cornea and then rebounds from the eye with a different velocity, which varies according to the IOP (Figure 12).
Figure 12. Disposable iCare probe.
The movement of the probe causes a voltage in the internal solenoid that is then amplified and digitally changed by a microprocessor. The IOP value is averaged from six consecutive measurements. The reliability of the final value is also displayed. The iCare tonometer is a reliable and precise instrument. It is rapid and easy to use, which is particularly helpful in busy clinics and with children, considering that there is no need for topic anesthesia [
82,
83,
84,
85]. The small surface contact makes it suitable to measure IOP after keratoplasty and in damaged corneas [
85,
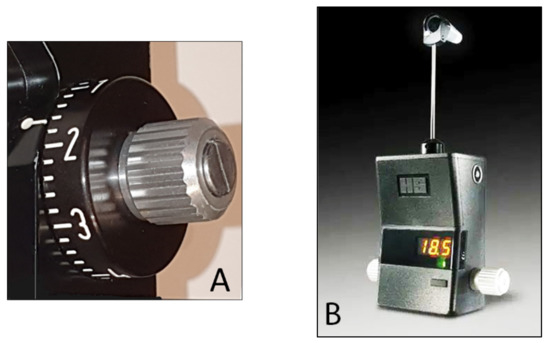
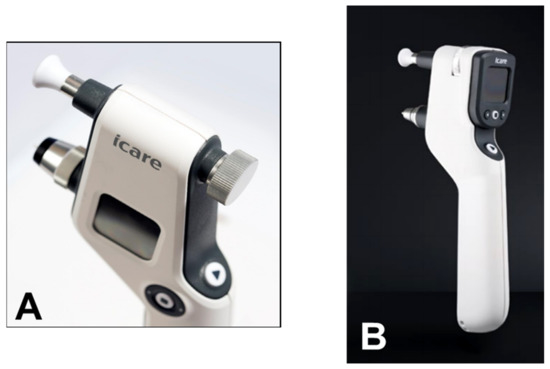
86]. The iCare PRO version released in 2011 uses a shorter probe, which can also be used to measure IOP in a supine position. The most recent versions of this instrument, which are updated versions of the iCare PRO with a long probe (iCare IC100 and IC200) (
Figure 13A,B), provide new features, such as a red or green light to show if the position of the probe is correct, in addition to providing the possibility of measuring IOP in a supine position [
87,
88].
Figure 13. (A) iCare 100; (B) iCare 200 version.
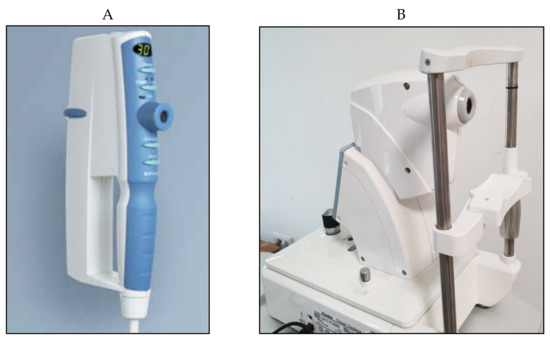
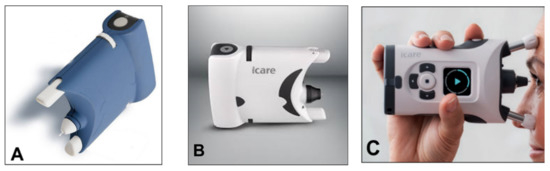
A simplified version (iCare One, replaced at first by the iCare Home and, recently, by the iCare Home2 (
Figure 14A–C)), which can autonomously be used by patients, has recently been introduced for at-home auto-tonometry. It can be helpful for detecting IOP peaks, especially in suspect glaucoma and in normal tension glaucoma subjects, when IOP measurements appear to be normal during office hours [
89,
90,
91,
92].
Figure 14. (A) iCare One; (B) iCare Home; (C) iCare Home2.
Numerous studies have compared the different versions of iCare with GAT and other non-conventional tonometers. When compared to gold standard GAT, clinical results report a good correlation of tonometry readings, with r values greater than 0.8 for low-to-moderate GAT readings [
93]. A recent study showed agreement between GAT readings and iCare to be good, with a <2 mmHg mean difference for all ranges of IOP [
87]. For IOP > 23 mmHg, rebound tonometry tends to underestimate IOP compared to GAT, showing readings that are significantly lower [
93].
7. Dynamic Contour Tonometry
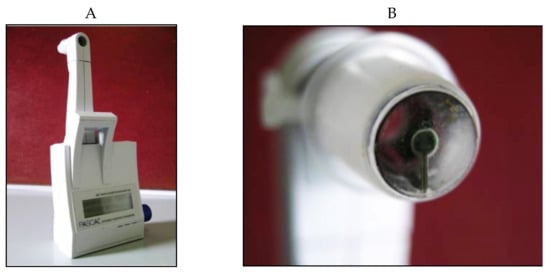
The Dynamic Contour Tonometer (PASCAL, DCT) (SMT Swiss Microtechnology AG, Port, Switzerland) is a relatively new device developed by Kaufmann et al. in 2003 [
105] and implemented by Kanngiesser et al. in 2005 [
106]. The DCT, which is not based on the applanation principle, calculates the IOP using the Pascal principle, according to which the pressure change is applied to all parts of a fluid in a contained enclosed space. The tonometer is positioned on the slit-lamp, requires the use of anesthetic drops (no fluorescein) and is automatically calibrated. It uses a concave contour tip that is equipped with a tiny sensor in the center of the contact surface (
Figure 15).
Figure 15. Dynamic Contour Pascal (A) with its sensor tip (B).
8. Applanation Resonance Tonometry
The Applanation Resonance Tonometer (ART), known in the current commercial version as BioResonator ART (BioResonator AB, Umea, Sweden) (
Figure 16), was developed by Eklund et al. in 2003 [
120]. It was released as both a manual and automatic version in 2012 [
121]. This tonometer uses the applanation tonometry principle combined with the resonance technique. The device needs must be mounted on a slit lamp, requires the use of local anesthetic drops before IOP measurement and uses a concave surface sensor tip, which is positioned on the cornea. The sensor tip is manually pushed towards the cornea in the manual version of the instrument, whereas the automatic version provides a tiny motor for movement of the tip. A resonance piezoelectric device is found in the tip of the sensor that generates a shift in frequency which is proportional to the area of contact. The IOP is based on the contact area and force measurement parameters, which are taken continuously throughout the test [
120,
121].
Figure 16. The BioResonator ART tonometer.
The ART probe must be carefully disinfected before each subject. This tonometer is self-calibrated and gives the repeated IOP measurement median and a quality index reflecting the standard deviation of the IOP values. The IOP measurement provided by the BioResonator ART is claimed to be more accurate than that of GAT considering that it represents the median of repeated measures; however, the precision of the instrument has been questioned [
122,
123,
124].
9. Continuous IOP Monitoring
All the aforementioned devices can be usefully employed for taking spot IOP measurements during office time. This can be acceptable in a screening setting, but, unfortunately, undetected elevated IOP spikes tend to occur during the night in many glaucomatous patients [
127]. IOP readings during clinical office hours fail to detect these peaks in more than 50% of cases with a significant underestimation of IOP [
128,
129,
130,
131]. Hughes et al. reported that data obtained with continuous monitoring in IOP using a 24 h device had an influence in therapeutic decisions in 79.3% of enrolled subjects [
132]. Keeping these data in mind, it can be inferred that our current standards in clinics with regard to taking IOP measurements may not suffice and thus need to be modified [
133].
An important step towards a more precise management of patients affected by ocular hypertension and chronic glaucoma would be the possibility of continuously monitoring IOP values not only during the day but also in the night, as occurs with the 24 h blood pressure Holter. This information could be particularly useful in the so-called normal tension glaucoma patients, which show significant damage progression despite an apparently normalized IOP. In these cases, an elevated IOP can sometimes be found during the night, especially early in the morning, outside office hours [
134,
135]. A number of devices, most of them only experimental, have been proposed for this purpose over the past 20 years [
136,
137,
138]. Some of them need to be surgically inserted into the eye, either during a cataract extraction procedure, usually embedded in an intraocular lens [
139,
140,
141], or positioned in the anterior chamber [
142,
143], or in the suprachoroidal space [
144]. A non-invasive continuous IOP measurement is also possible using special contact lenses with different types of miniaturized sensors and a wireless power transmission of data to a recorder.
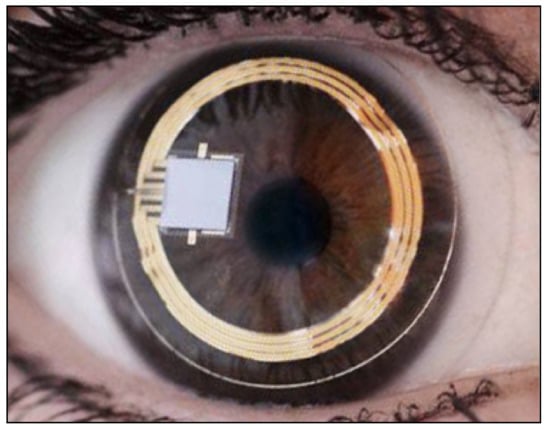
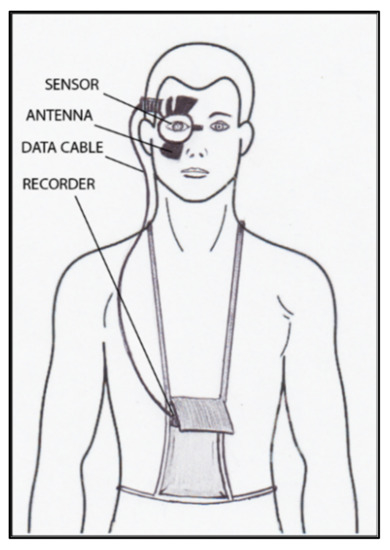
The contact lens sensor Sensimed Triggerfish (Triggerfish CLS, Sensimed AG, Lausanne, Switzerland) is a miniaturized electromechanical system with a microprocessor embedded in a disposable silicon contact lens, which transmits a signal to an external wireless antenna located in the periocular surface (
Figure 17 and
Figure 18). The data are then transferred to a portable recorder, for a total of 288 data sets in 24 h. This device can measure small modifications in the curvature of the cornea believed to be due to variations of IOP [
145,
146,
147,
148,
149].
Figure 17. Sensimed Triggerfish.
Figure 18. Schematic view of Triggerfish, wireless antenna and portable recorder.
Triggerfish CLS is usually well tolerated [
150,
151,
152] and has also been shown to have high reproducibility [
150,
151,
152,
153,
154,
155]. The information obtained with these device parameters might be useful in assessing changes and IOP fluctuations in subjects with pseudoexfoliation syndrome, pigment dispersion, and in predicting the visual field loss progression rate [
156,
157]. Several studies have shown the usefulness of this contact lens sensor for assessment of the risk of glaucoma, which may prove to be important in subjects with NTG, in which IOP tends to be normal with diurnal readings [
158,
159,
160,
161].
The main problem with this device is that there is no direct correlation between corneal changes, expressed in millivolt equivalent (mVeq), and IOP values. Studies have shown that IOP measurements taken with GAT and Triggerfish values tend to have a high correlation at the beginning, after the insertion of CLS [
148]; however, the correlation becomes poor after 24 h [
153,
154].
CLS is advantageous because it is not invasive, can be easily removed and dismantled [
155], readily available [
155], accepted and tolerated by patients [
150,
151,
152], and provides good reproducibility [
151,
153,
154]. The validity (i.e., considering the estimation accuracy of IOP readings) and relatively costly equipment of CLS are important drawbacks, which render the clinical usefulness of this instrument still debatable in literature [
153,
154].
Other types of devices able to measure IOP, either implantable or non-invasive [
162,
163,
164,
165,
166,
167,
168,
169,
170,
171,
172,
173], have been proposed, but almost all are still experimental and need further studies before being introduced into clinical practice.