Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
There are over fifty examples in which the inner coordination spheres about the Pt(II) atoms of the Pt(η3-X3L)(PL) type are formed by variable combinations of donor atoms of tridentate ligands. Each η3-ligand creates two metallocyclic rings. The complexes based on membered metallocyclic rings can be divided into four groups: 1. 6+6-Membered Metallocyclic Rings, 2. 6+5-Membered Metallocyclic Rings, 3. 5+6-Membered Metallocyclic Rings, and 4. 5+5-Membered Metallocyclic Rings.
- structure
- Pt(η3-X3L)(PL)
- distortion
- trans-effect
1. 6+6-Membered Metallocyclic Rings
There are only three examples in which a η3-ligand creates such rings (Table 1). In [Pt(η3-C22H11F6N3O2–O1,N1,O2)(PPh3)] (at 173 K) [1], the η3-ligand forms a metallocyclic ring of the O1C3N1C3O2 type with common ligating N1 atoms. The values of the chelate L-Pt-L angles are 90.6° (O1-Pt-N1) and 90.2° (N1-Pt-O2). The O1C2NN1C3O2 type with the respective chelate angles of 88.2° (O1-Pt-N1) and 90.0° (N1-Pt-O2) was found in [Pt(η3-C14H10N2O3–O1,N1,O2)(PPh3)] (at 150 K) [2]. The remaining L-Pt-L angles open in the following order (mean values): 88.1° (O2-Pt-P) < 89.0° (O1-Pt-P) < 176.0° (N1-Pt-P) < 177.7° (O1-Pt-O2). The monodentate PPh3 displayed square-planar geometry about each Pt(II) atom. The Pt-L bond distance increased in the following order (mean values): 1.995 Å (Pt-O1 trans to O2) < 1.996 Å (Pt-O2) < 2.010 Å (Pt-N1) < 2.254 Å (Pt-P).
Table 1. Structural data for Pt(η3-X3)(Y) derivatives. a—6+6-membered metallocyclic rings.
| Complex | Chromophore Chelate Rings τ4 b |
Pt -L c (Å) |
L-Pt-L c (°) |
Ref. |
|---|---|---|---|---|
| [Pt(η3-C22H11F6N3O2-O1,N1,O2)(PPh3)] (at 173 K) |
PtO1N1O2P (O1C3N1C3O2) 0.032 |
O1 1.994(2) N1 2.021(2) O2 2.004(2) P 2.256(2) |
O1,N1 90.6 d N1,O2 90.2 d O1,O2 179.0 O1,P 90.6 O2,P 87.5 N1,P 177.0 |
[1] |
| [Pt(η3-C14H10N2O3-O1,N1,O2)(PPh3)] (at 150 K) |
PtO1N1O2P (O1C2NN1C3O2) 0.024 |
O1 1.995(2) N1 2.000(2) O2 1.988(2) P 2.251(2) |
O1,N1 88.2 d N1,O2 90.0 d O1,O2 176.5 O1,P 89.0 O2,P 90.7 N1,P 175.0 |
[2] |
| [Pt{η3-C12H24S3-S1,S2,S3}(PPh3)]BF4 | PtS1S2S3P (S1C3S2C3S3) 0.035 |
S1 2.330(2) S2 2.339(2) S3 2.336(2) P 2.332(2) |
S1,S2 87.1(2) d S2,S3 89.5(2) d S1,S3 176.3(2) S1,P 91.1(2) S3,P 92.3(1) S2,P 171.0(2) |
[3] |
(a) Where more than one chemically equivalent distance or angle is present, the mean value is tabulated. The number in parentheses is the e.s.d. (b) Parameter τ4, degree of distortion. (c) The chemical identity of the coordinated atom/ligand is specific to these columns. (d) Six-membered metallocyclic ring.
For the complex [Pt{η3-C12H24S3-S1,S2,S3}(PPh3)]BF4, the η3-ligand creates a pair of six-membered metallocyclic rings of the S1C3S2C3S3 type (as shown in Figure 1) [3]. The values of the chelate angles are 87.1° (S1-Pt-S2) and 89.5° (S2-Pt-S3). The remaining L-Pt-L bond angles open in the following order: 91.1° (S1-Pt-P) < 92.3° (S3-Pt-P) < 171.0° (S2-Pt-P) < 176.3° (S1-Pt-S3). The Pt-L bond distance increases in the following order: 2.330 Å (Pt-S1) < 2.332 Å (Pt-P) < 2.336 Å (Pt-S3) < 2.339 Å (Pt-S2 trans to P). Noticeably, the trans-X1-Pt-X3 bond angles are somewhat bigger than the trans-X2-Pt-P bond angles (Table 1).
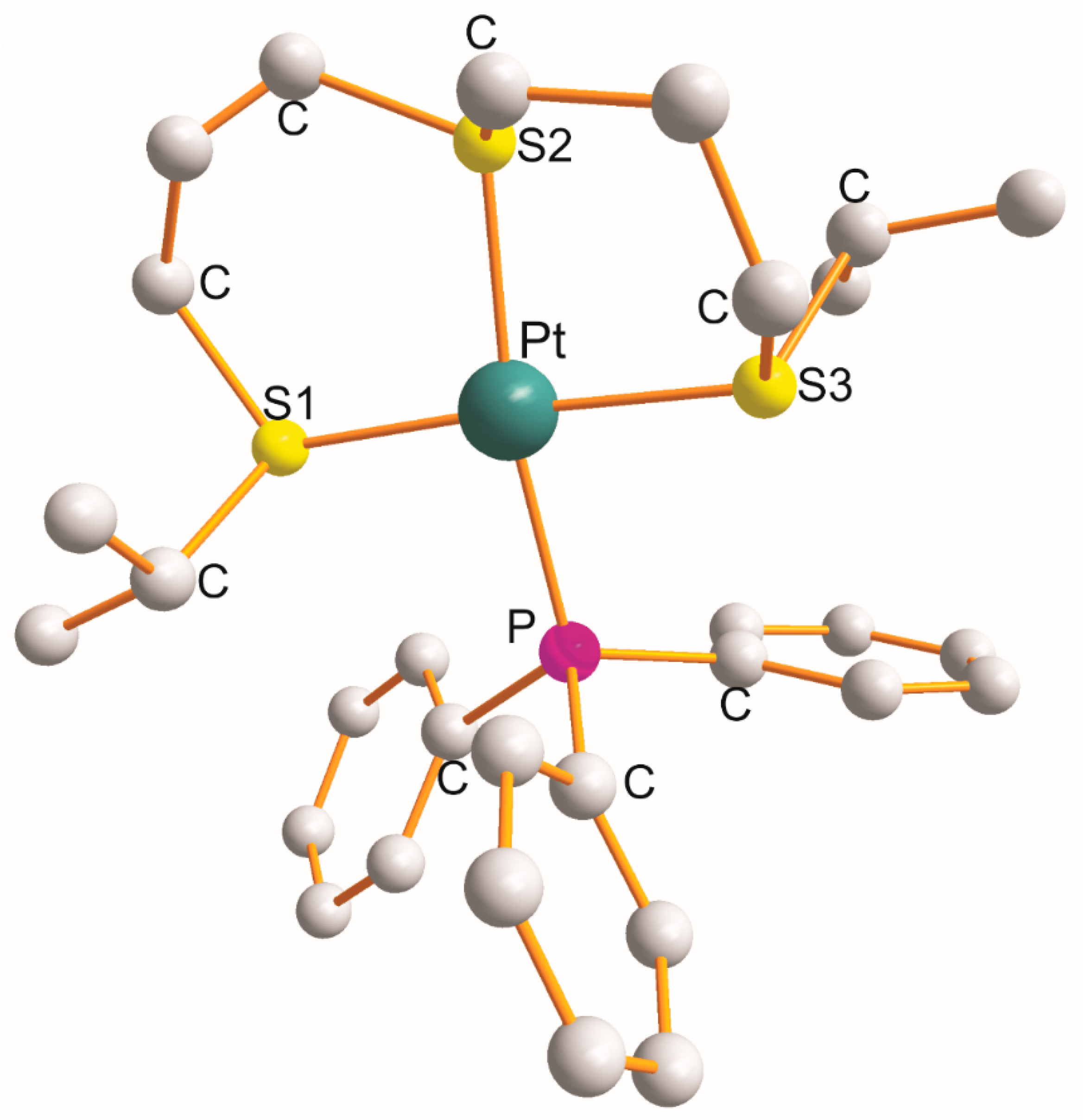
Figure 1. Structure of [Pt{η3-C12H24S3-S1,S2,S3}(PPh3)] [3].
2. 6+5-Membered Metallocyclic Rings
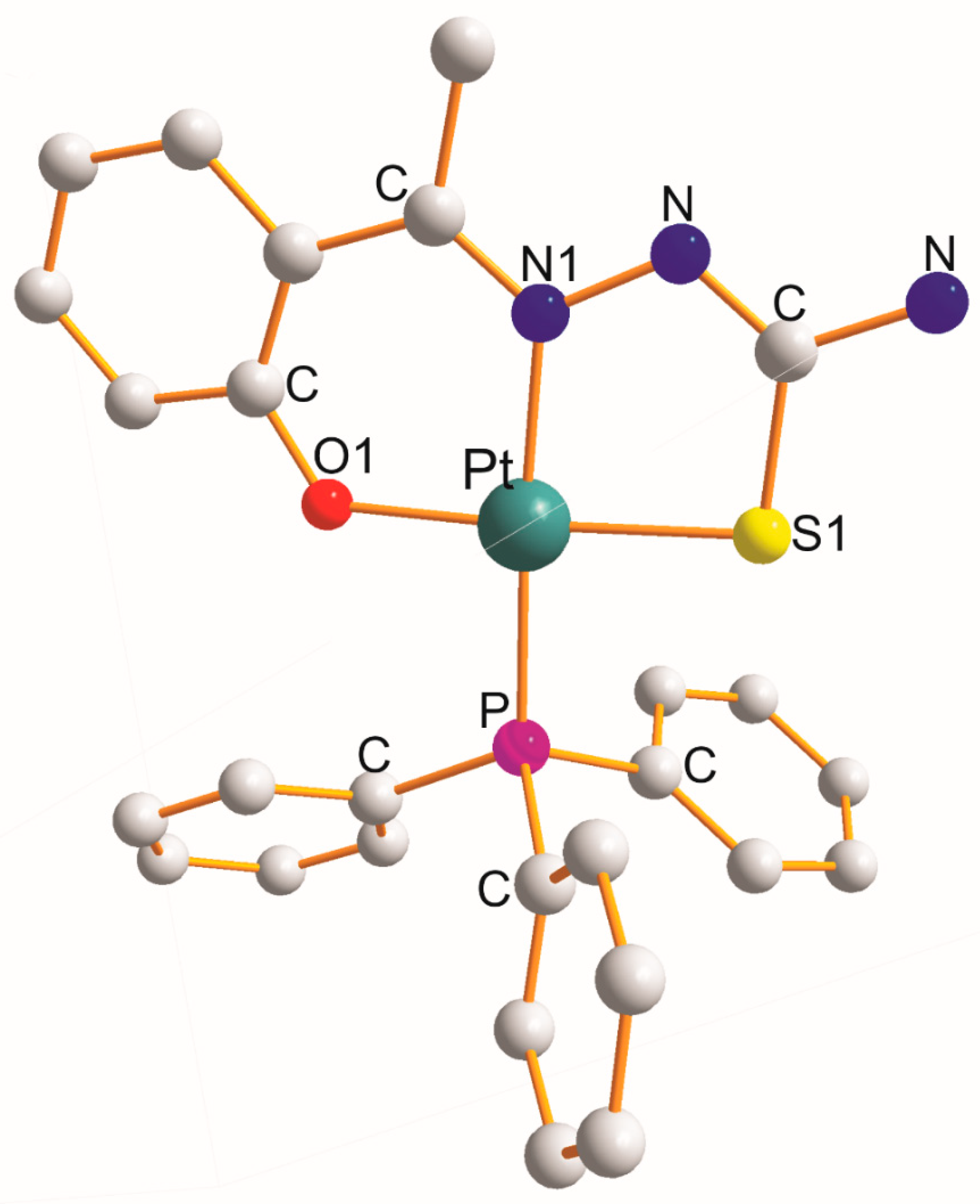
There are five examples namely [Pt(η3-C16H14N2OS2–O1,N1,S1)(PPh3)] [4], [Pt(η3-C16H13N3O3S2–O1,N1,S1)(PPh3)] (at 200K) [4], [Pt(η3-C8H8N3OS–O1,N1,S1)(PPh3)] toluene [5], [Pt(η3-C9H9N3OS–O1,N1,S1)(PPh3)] (at 100 K) (Figure 2) [6], and [Pt(η3-C18H16N2OS2–O1,N1,S1)(PPh3)] [4] (Table 2). In each of them, the η3-ligand creates six- and five-membered metallocyclic rings with a common ligating N1 atom of the O1C3N1NCS1 type. The values of the respective chelate angles (mean values) are 92.3° (O1-Pt-N1) and 84.6° (N1-Pt-S1). The remaining L-Pt-L bond angles open in the following order (mean values): 90.7° (O1-Pt-P) < 92.4° (S1-Pt-P) < 175.8° (N1-Pt-P) < 175.9° (O1-Pt-S1). Interestingly, the mean values of both trans-O1-Pt-S1 and N1-Pt-P angles are equal. The Pt-L bond distance increases (mean values) in the following order: 2.028 Å (Pt-O1 trans to S1) < 2.035 Å (Pt-N1 trans to P) < 2.244 Å (Pt-S1) < 2.259 Å (Pt-P).

Figure 2. Structure of [Pt(η3-C9H9N3OS–O1,N1,S1)(PPh3)] [6].
Table 2. Structural data for Pt(η3-X3)(Y) derivatives. a—6+5-membered metallocyclic rings.
| Complex | Chromophore Chelate Rings τ4 b |
Pt -L c (Å) |
L-Pt-L c (°) |
Ref. |
|---|---|---|---|---|
| [Pt(η3-C16H14N2OS2-O1,N1,S1)(PPh3)] | PtO1N1S1P (O1C3N1NCS1) 0.016 |
O1 1.992 N1 2.034 S1 2.245 P 2.258 |
O1,N1 91.2 d N1,S1 85.0 e O1,S1 176.0 O1,P 89.0 S1,P 93.1 N1,P 178.1 |
[4] |
| [Pt(η3-C16H13N3O3S2-O1,N1,S1)(PPh3)] (at 200 K) |
PtO1N1S1P (O1C3N1NCS1) 0.018 |
O1 2.001 N1 2.041 S1 2.239 P 2.248 |
O1,N1 92.6 d N1,S1 85.3 e O1,S1 177.6 O1,P 89.0 S1,P 93.3 N1,P 176.0 |
[4] |
| [Pt(η3-C8H8N3OS-O1,N1,S1)(PPh3)].toluene | PtO1N1S1P (O1C3N1NCS1) 0.020 |
O1 2.015 N1 2.031 S1 2.234 P 2.257 |
O1,N1 93.1 d N1,S1 83.8 e O1,S1 176.6 O1,P 89.9 S1,P 93.3 N1,P 176.3 |
[5] |
| [Pt(η3-C9H9N3OS-O1,N1,S1)(PPh3)] (at 103 K) |
PtO1N1S1P (O1C3N1NCS1) 0.024 |
O1 2.085 N1 2.036 S1 2.257 P 2.260 |
O1,N1 92.5 d N1,S1 85.1 e O1,S1 175.7 O1,P 91.5 S1,P 91.0 N1,P 175.6 |
[6] |
| [Pt(η3-C18H16N2OS2-O1,N1,S1)(PPh3)] | PtO1N1S1P (O1C3N1NCS1) 0.037 |
O1 2.045 N1 2.029 S1 2.246 P 2.269 |
O1,N1 92.3 d N1,S1 83.2 e O1,S1 173.6 O1,P 93.1 S1,P 91.2 N1,P 173.1 |
[4] |
(a) Where more than one chemically equivalent distance or angle is present, the mean value is tabulated. The number in parentheses is the e.s.d. (b) Parameter τ4, degree of distortion. (c) The chemical identity of the coordinated atom/ligand is specific to these columns. (d) Six-membered metallocyclic ring. (e) Five-membered metallocyclic ring.
3. 5+6-Membered Metallocyclic Rings
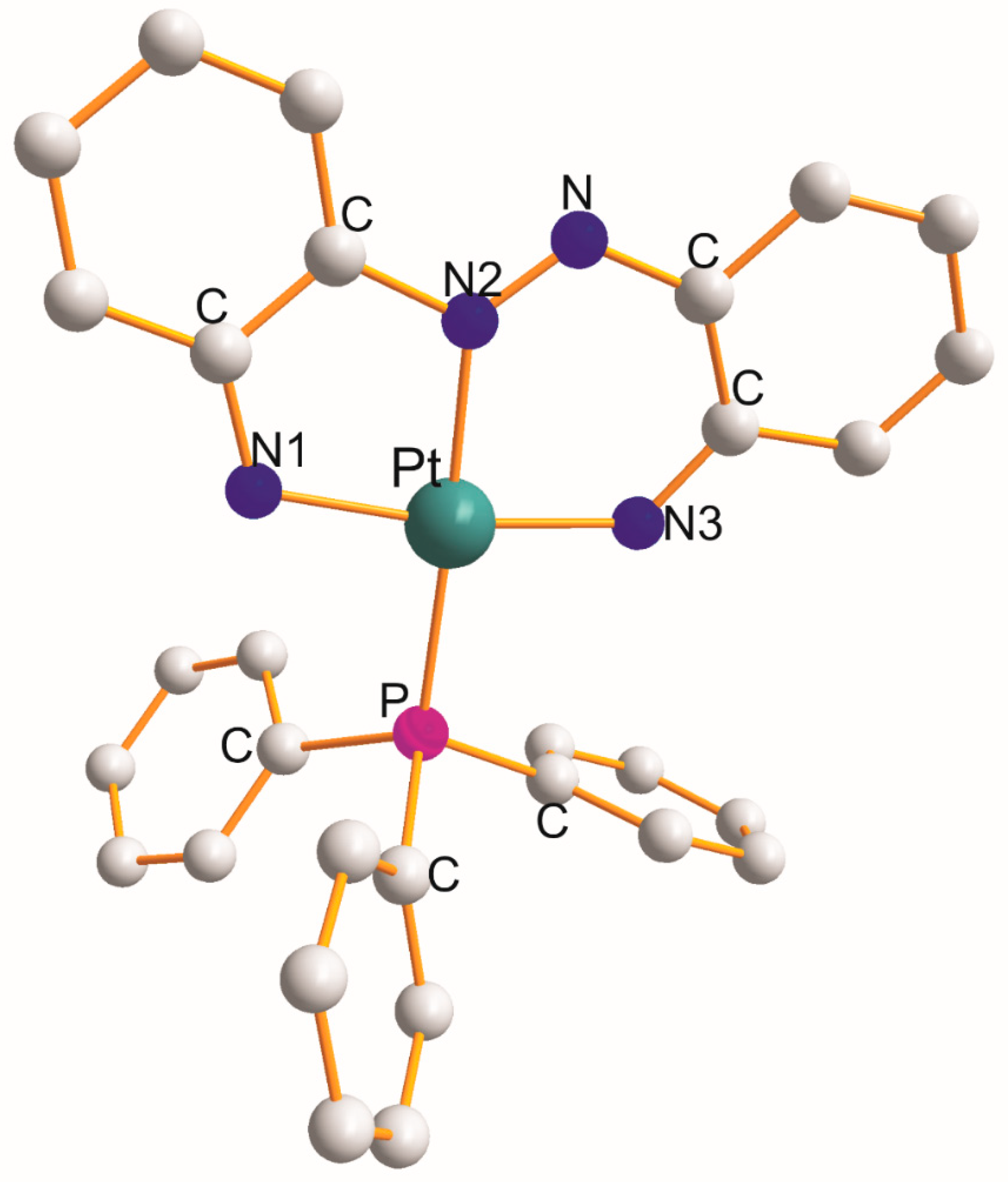
There are four complexes mentioned in this section, namely [Pt(η3-C12H10N4–N1,N2,N3)(PPh3)] (at 100 K) [7], [Pt(η3-C13H9NO2–O1,N1,O2)(PPh3)] [8], [Pt(η3-C12H16N2O4Se2–Se1,N1,Se2){P(η1-C11H19O5)(Ph)2}] [9], and [Pt(η3-C29H20F6S2O–S1,S2,O1)(PPh3)] (at 100 K) [10], and their structural parameters are gathered in Table 3. The structure of [Pt(η3-C12H10N4–N1,N2,N3)(PPh3)] [7] is shown in Figure 3 as an example. Each η3-ligand creates five and six metallocyclic rings. The donor atoms of the respective η3-ligands play a role in the size of the L-Pt-L chelate angles. These angles increase in the following sequences:

Figure 3. Structure of [Pt(η3-C12H10N4–N1,N2,N3)(PPh3)] [7].
Table 3. Structural data for Pt(η3-X3)(Y) derivatives. a—5+6-membered metallocyclic rings.
| Complex | Chromophore Chelate Rings τ4 b |
Pt -L c (Å) |
L-Pt-L c (°) |
Ref. |
|---|---|---|---|---|
| [Pt(η3-C12H10N4-N1,N2,N3)(PPh3)] (at 100 K) |
Pt N1N2N3P (N1C2N2NC2N3) 0.034 |
N1 1.984 N2 2.025 N3 1.964 P 2.255 |
N1,N2 81.7 e N2,N3 89.6 d N1,N3 170.6 N1,P 93.0 N3,P 96.3 N2,P 177.2 |
[7] |
| [Pt(η3-C13H9NO2-O1,N1,O2)(PPh3)] | Pt O1N1O2P (O1C2N1C3O2) 0.034 |
O1 1.975(9) N1 2.064(12) O2 1.996(9) P 2.248 |
O1,N1 82.4(4) e N1,O2 94.8(4) d O1,O2 176.4(4) O1,P 91.5(3) O2,P 91.5(3) N1,P 172.4 |
[8] |
| [Pt(η3-C12H16N2O4Se2-Se1,N1,Se2){P(η1-C11H19O5)(Ph)2}] | Pt Se1N1Se2 (Se1C2N1NC2Se2) 0.036 |
Se1 2.394 N1 2.078 Se2 2.349 P 2.259 |
Se1,N1 83.3 e N1,Se2 98.3 d Se1,Se3 176.3 Se1,P 87.2 Se2,P 90.7 N1,P 170.9 |
[9] |
| [Pt(η3-C29H20F6O4S2O-S1,S2,O1)(PPh3)] (at 100 K) |
Pt S1S2O1P (S1C2S2C3O1) 0.059 |
S1 2.268 S2 2.277 O1 2.066 P 2.253 |
S1,S2 90.2 e S1,O1 99.2 d S1,O1 169.6 S1,P 89.2 O1,P 99.2 S2P 169.4 |
[10] |
(a) Where more than one chemically equivalent distance or angle is present, the mean value is tabulated. The number in parentheses is the e.s.d. (b) Parameter τ4, degree of distortion. (c) The chemical identity of the coordinated atom/ligand is specific to these columns. (d) Six-membered metallocyclic ring. (e) Five-membered metallocyclic ring.
N1C2N2NC2N3—81.7° (N1-Pt-N2) and 89.6° (N2-Pt-N3);
O1C2N1C3O2—2.4° (O1-Pt-N1) and 94.8° (N1-Pt-O2);
Se1C2N1NC2Se2—83.3° (Se1-Pt-N1) and 98.3° (N1-Pt-Se2);
S1C2S2C3O1—90.2° (S1-Pt-S2) and 99.2° (S2-Pt-O1).
The monodentate PL displayed distorted square-planar geometry about Pt(II) atoms. The Pt-L bond distance to PL increased in the following order: 2.025 Å (Pt-N2) < 2.064 Å (Pt-N1) < 2.078 Å (Pt-N1) < 2.277 Å (Pt-S2). The order follows the above-mentioned sentence for the Pt-L (L is a common central ligating atom between five and six-rings).
4. 5+5-Membered Metallocyclic Rings
There are thirty-nine compounds in which each η3-ligand creates two five-membered metallocyclic rings. These complexes based on variable combinations of atoms involved in the chelate angles can be divided into twelve groups.
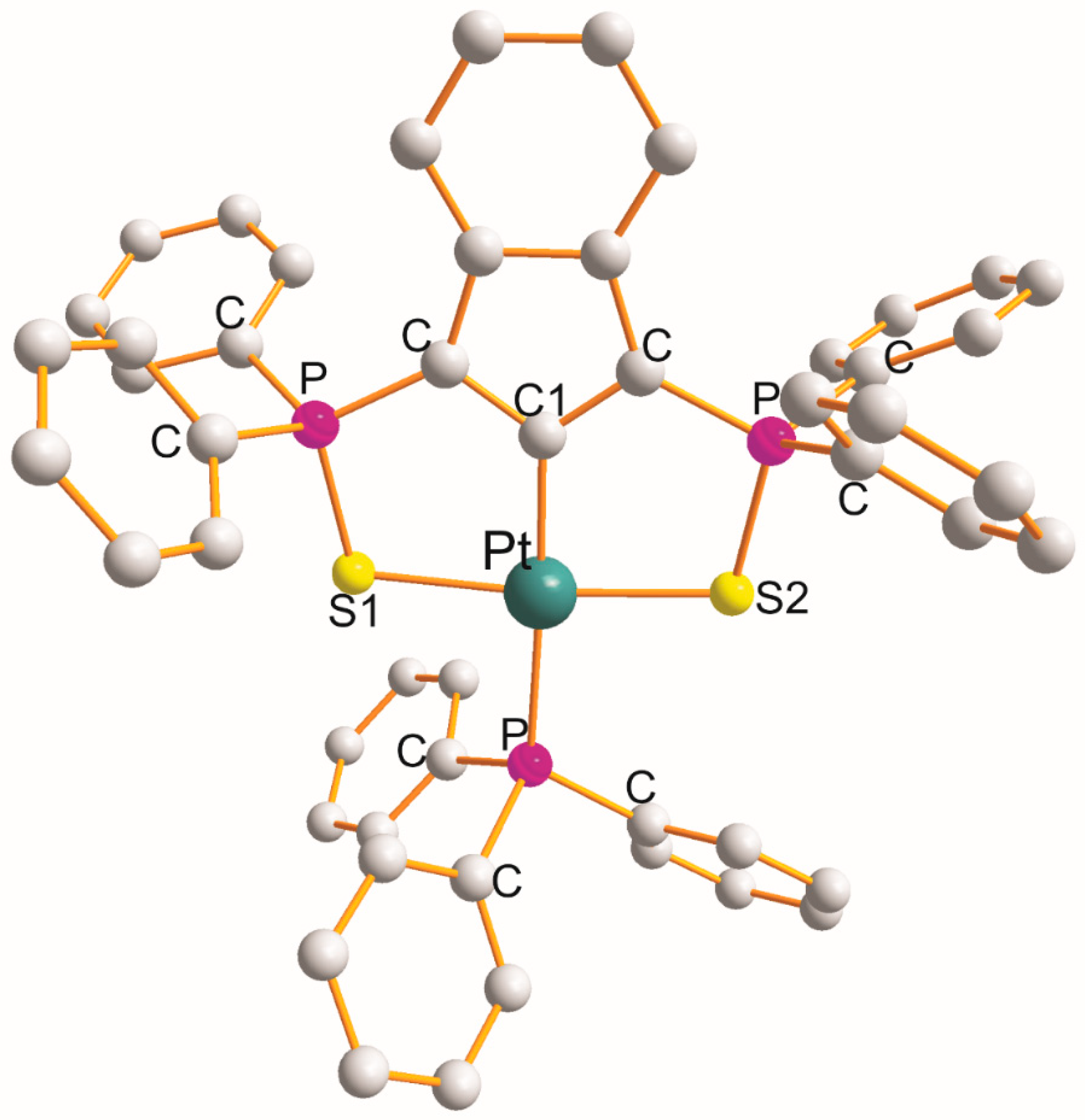
The structure of [Pt(η3-C33H24P2S2–S1,C1,S2)(PPh3)].CH2Cl2 [11] is shown in Figure 4. The η3-ligand creates two five-membered metallocyclic rings with a common C1 atom of the S1PCC1CPS2 type with chelate angles of 87.9° (S1-Pt-C1) and 87.7° (C1-Pt-S2). This is the only example of this type. The PPh3 demonstrated distorted square-planar geometry about Pt(II) atoms. The remaining L-Pt-L bond angles open in the following order: 89.7° (S1-Pt-P) < 94.2° (S2-Pt-P) < 173.8° (S1-Pt-S2) < 176.9° (C1-Pt-P). The Pt-L bond distance increases in the following order: 2.020 Å (Pt-C1) < 2.316 Å (Pt-S2) < 2.332 Å (Pt-S1) < 2.322 Å (Pt-P).

Figure 4. Structure of [Pt(η3-C33H24P2S2–S1,C1,S2)(PPh3)] [11].
In another two complexes, namely [Pt(η3-C12H12N2Te3–Te1,Te2,Te3)(PPh3)].C6H6 and [Pt(η3-C10H8N2Te3–Te1,Te2,Te3)(PPh3)] [12], which are isostructural, the η3-ligand creates a pair of five-membered metallocyclic rings with common central ligating Te2 atoms of the Te1CNTe2NCTe3 type. The mean values of the respective angles are 92.2 (±6)° (Te1-Pt-Te2) and 92.0 (±6)° (Te2-Pt-Te3). The PPh3 ligand demonstrated distorted square-planar geometry about each Pt(II) atom. The remaining L-Pt-L bond angles open in the following order (mean values): 88.1 (±1.2)° (Te3-Pt-P) ~ 88.1 (±2.31)° (Te1-Pt-P) < 173.3 (±8)° (Te1-Pt-Te3) < 173.4 (±2.1)° (Te2-Pt-P). The Pt-L bond distance increases in the following order (mean values): 2.283 (±1) Å (Pt-P) < 2.571 (±2) Å (Pt-Te2) < 2.591 (±3) Å (Pt-Te1) < 2.592 (±20) Å (Pt-Te3).
In another two complexes, namely Pt(η3-C12H9N2S2B–S1,B1,S2)(PPh3)].0.06CH2Cl2 [13] and Pt(η3-C13H14N5S3B–S1,B1,S2)(PPh3)] [14], each η3-ligand creates a pair of five-membered metallocyclic rings with a common central ligating B1 atom of the S1CNB1NCS2 type. The values of the respective chelate angles are (mean values): 80.4 (±6)° (S1-Pt-B1) and 85.7 (±8)° (B1-Pt-S2). The PPh3 demonstrated distorted square-planar geometry about the Pt(II) atom. The remaining L-Pt-L bond angles open in the following order (mean values): 95.9 (±5)° (S2-Pt-P) < 99.3 (±5)° (S1-Pt-P) < 162.4 (±1.0)° (S1-Pt-S2) < 174.4 (±2.2)° (B1-Pt-P). The Pt-L bond distance increases in the following order (mean values): 2.110 (±19) Å (Pt-B1) < 2.284 (±10) Å (Pt-S2) < 2.301 (±3) Å (Pt-S1) < 2.382 (±2) Å (Pt-P).
This entry is adapted from the peer-reviewed paper 10.3390/cryst13040599
References
- Dahm, G.; Borré, E.; Fu, C.; Bellemin-Laponnaz, S.; Mauro, M. Tridentate Complexes of Palladium(II) and Platinum(II) Bearingbis-Aryloxide Triazole Ligands: A Joint Experimental and Theoretical Investigation. Chem. Asian J. 2015, 10, 2368–2379.
- Halder, S.; Drew, M.G.B.; Bhattacharya, S. Palladium and platinum complexes of 2-(2′-carboxyphenylazo)-4-methylphenol: Synthesis, structure and spectral properties. J. Chem. Sci. 2008, 120, 441–446.
- Loeb, S.; Mansfield, J.R. Platinum(II) complexes of the tridentate thioether ligands RS(CH2)3S(CH2)3SR (R = Et, iPr, Ph). Structures of , , and
- Maia, P.I.D.S.; Fernandes, A.G.D.A.; Silva, J.J.N.; Andricopulo, A.D.; Lemos, S.S.; Lang, E.S.; Abram, U.; Deflon, V.M. Dithiocarbazate complexes with the 2+ (M = Pd or Pt) moiety: Synthesis, characterization and anti-Tripanosoma cruzi activity. J. Inorg. Biochem. 2010, 104, 1276–1282.
- Lobana, T.S.; Bawa, G.; Hundal, G.; Zeller, M. The Influence of Substituents at C2 Carbon Atom of Thiosemicarbazones on their Dentacy in PtII/PdII Complexes: Synthesis, Spectroscopy, and Crystal Structures. Z. Anorg. Allg. Chem. 2008, 634, 931–937.
- Halder, S.; Butcher, R.J.; Bhattacharya, S. Synthesis, structure and spectroscopic properties of some thiosemicarbazone complexes of platinum. Polyhedron 2007, 26, 2741–2748.
- Mandal, P.; Lin, C.-H.; Brandão, P.; Mal, D.; Felix, V.; Pratihar, J.L. Synthesis, characterization, structure and catalytic activity of (NNN) tridentate azo-imine nickel(II), palladium(II) and platinum(II) complexes. Polyhedron 2016, 106, 171–177.
- Motschi, H.; Nussbaumer, C.; Pregosin, P.S.; Bachechi, F.; Mura, P.; Zambonelli, L. Thetrans-Influence in Platinum (II) Complexes.1H- and13C-NMR. and X-ray structural studies of tridentateSchiff’s base complexes of platinum (II). Helv. Chim. Acta 1980, 63, 2071–2086.
- Arsenyan, P.; Oberte, K.; Rubina, K.; Belyakov, S. Synthesis and application of a new selenoplatinum catalyst. Tetrahedron Lett. 2005, 46, 1001–1003.
- Kano, N.; Kusaka, S.; Kawashima, T. Insertion of platinum and palladium into a sulfur(IV)–sulfur(II) bond of a sulfur-substituted sulfurane. Dalton Trans. 2010, 39, 456–460.
- Monot, J.; Merceron-Saffon, N.; Martin-Vaca, B.; Bourissou, D. SCS indenediide pincer complexes: Zr to Pd and Pt transmetallation. J. Organomet. Chem. 2017, 829, 37–41.
- Chauhan, R.S.; Kedarnath, G.; Wadawale, A.; Muñoz-Castro, A.; Arratia-Perez, R.; Jain, V.K.; Kaim, W. Tellurium(0) as a Ligand: Synthesis and Characterization of 2-Pyridyltellurolates of Platinum(II) and Structures of (R = H or Me). Inorg. Chem. 2010, 49, 4179–4185.
- Zech, A.; Haddow, M.F.; Othman, H.; Owen, G.R. Utilizing the 8-Methoxycyclooct-4-en-1-ide Unit As a Hydrogen Atom Acceptor en Route to “Metal–Borane Pincers”. Organometallics 2012, 31, 6753–6760.
- Owen, G.R.; Gould, P.H.; Hamilton, A.; Tsoureas, N. Unexpected pincer-type coordination (κ3-SBS) within a zerovalent platinum metallaboratrane complex. Dalton Trans. 2010, 39, 49–52.
This entry is offline, you can click here to edit this entry!
