Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The environmental assessment considers greenhouse gaseous emissions as a comparative parameter for different thermochemical processes. Several approaches exist for measuring greenhouse gaseous emissions, mainly CO, CO2, N2O, and CH4, from thermochemical reactors. This text recommends monitoring equipment and techniques to monitor and eliminate greenhouse gaseous emissions in thermochemical reaction such as pyrolysis and gasification.
- carbon monoxide
- carbon dioxide
- methane
- syngas
- gas analyzer
- biomass
- combustion
- thermogravimetric analyzer
- pyrolysis
1. Introduction
Monitoring equipment is used in research and development when the environmental footprint and emissions calculations make a significant impact on the process plant design, plant sizing, cost structure, and future marketing of the process technology [1]. Life cycle assessment strategies evaluate the environmental impact of greenhouse gaseous (GHG) emissions and ensure that the proposed process design complies with the environmental emission standards and regulations set by environmental agencies [2]. Life cycle assessment (LCA) also provides designers, environmental agencies, and engineers with options that are used in decision-making in different parts of the project, including the preliminary design, construction, or execution of chemical plants. LCA strategies are used in businesses to optimize spending and comply with environmental regulations, as well as to compare alternative technologies in terms of spending and the carbon footprint of different process routes [3][4][5].
Thermochemical processes convert solid and plastic waste deposits, recover thermal energy, and generate electricity, as well as reduce environmental and health impacts [6][7].
2. Environmental Assessment of Greenhouse Gas Emissions from Thermochemical Reactions
Several approaches exist for measuring greenhouse gaseous emissions, mainly CO, CO2, N2O, and CH4, from thermochemical reactors. Important factors to determine the quality of the flux measurements from thermochemical reactors are the collected gaseous samples for these reactors [8][9][10]. Thermochemical reactions, such as pyrolysis and gasification, burn biomass or solid waste, with insufficient oxygen supply under stoichiometric conditions to produce combustible gaseous products, referred to as syngas. These thermochemical processes are recommended due to the reduced release of toxins, as well CO and methane, compared to those released from the combustion process. In gasifiers, the air-to-fuel ratio varies between 5:1 and 8:1, while the required ratio for combustion is 3:1 [4][8][9][11] There are several methods for controlling and reducing greenhouse gaseous emissions from thermochemical reactions, including increasing energy efficiency, the switching of fuel, heat integration, and the use of more efficient methods, such as heat exchanger networks and the catalytic conversion of NOx and CO emissions [4][11][12].
The controlled variables are variables that remain constant throughout the reaction, ensuring accurate temperature profiles. Controlled variables are kept constant, so they do not influence the reaction outcomes. Controlled variables could be the agitation rate, feedstock rate, nitrogen supply rate, and reflux ratio [13]. The manipulated variables are variables that are controlled, and this change is based on feedback signals, such as thermal plasma or inductive heater current, product withdrawal flow rate, and cooling water flow rate [14][15][16][17].
The collected solid waste may contain several components such as organic and decomposable materials that might require separation before the combustion process. Organic and decomposable materials are recommended to be sent to pyrolyzers and gasifiers. Unprocessed products from rectors, such as tar, ash, and char, are sent to landfills, as shown below in Figure 1 [18]:
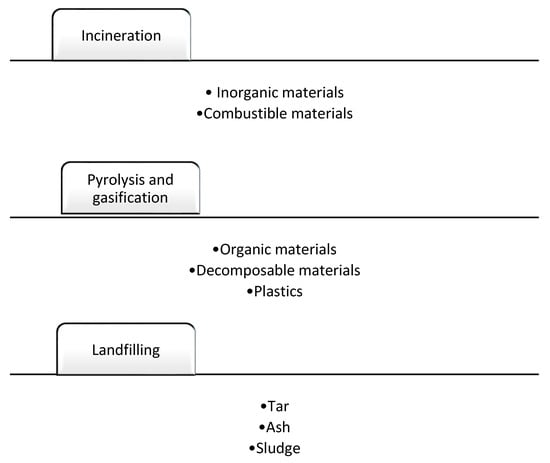
Figure 1. Recommended MSW treatment practices for different feedstocks [18].
As seen in Figure 1, organic materials are suitable for pyrolyzers or gasifiers due to their easy conversion and thermal cracking. Incinerators require the separation of incombustible to avoid heat loss and the formation of agglomerates. Landfilling as the last recycling strategy is recommended for slag, unprocessed waste, and other materials that cannot be processed in chemical recycling processes [19]. According to EU regulations, a reduction of 65% is required in landfilling facilities [15][16][20]. In a standard landfilling chemical plant, the following process stages are required [21]:
-
Municipal solid waste processing.
-
A gas separation and processing unit.
-
An environmental control and monitoring unit.
-
A gas and steam combustion unit.
-
A steam generation unit.
-
A waste to energy process system.
-
A heat integration unit.
The thermochemical processes have limiting factors, such as high thermal energy consumption, high environmental impact, and low thermal efficiency, as well as the release of greenhouse gas emissions [18]. Optimal boiler conditions and turbine efficiency are required for high-energy generation. Incineration is the full combustion of heterogenous combustible matter, in excess oxygen, of organic and inorganic matter, including minerals with the highest allowable water vapor content of 35 wt.% and an optimal moisture content of around 15 wt.%. Below are the main process stages in incinerators:
Drying and degassing stage: This stage prepares municipal solid waste with optimal moisture content and surface area to ensure optimum heat transfer and low water content, which helps improve energy efficiency. This stage reduces also the PSD (particle size diameter) of MSW feedstock, which aids in heat transfer and helps avoid agglomeration and slug formation.
Incinerator stage: A thermal cracking process of MSW, in excess oxygen, releasing thermal energy at 800 °C to 1000 °C [22][23]. The solid waste volume is massively reduced, and the volume of undesired products, including tar and char content, is minimized. Incinerators usually contain several heat zones and two air supply sources to ensure the complete combustion of combustible solid waste materials. The released combustible gas contains dioxins, furans, nitrogen oxides, carbon monoxide, and oxygen in controllable levels, depending on the municipal solid waste mass composition. Excess oxygen is supplied to ensure a complete combustion process, based on stoichiometric calculations. Incinerators adapt different grate designs and heat transfer surface areas, depending on the MSW feedstock and the heat exchanging network (HEN).
Flue gas scrubbers: This stage focuses on the removal of slug, as well as unprocessed waste and heavy metal contaminants, during the incineration process to ensure that the incinerator complies with environmental standards before releasing flue gas into the atmosphere.
Boiler and steam generation stage: Combustible gases, including syngas and light hydrocarbon gases, are burnt using a gas and steam turbine to optimize steam and electricity generation. The steam is generated in a heat recovery network using flue gas and a steam generation cycle.
The incineration quality is determined by the degree of complete combustion, which could be measured by the mass percentage of CO, CO2, and NOx, since complete combustion requires negligible carbon monoxide levels below 5 ppm [18]. The residence time of solid waste incinerators is from several minutes to one hour, based on the mass composition of the solid waste feedstock and the process temperatures used [24]. Incinerators ensure minimum combustion temperatures and minimum residence time to ensure full combustion in the primary and secondary air zones, including excess oxygen supply [18].
Boilers integrated in incinerators could have different designs including vertical or horizontal setups with different oxygen levels [24]. A typical incinerator steam generation network is divided into a superheater, an economizer, and an evaporator. Pyrolysis requires inert conditions provided by a nitrogen or argon gas supply at elevated temperatures. Thus, the following equation illustrates the thermal cracking and energy generation process of incinerators [24]:
CnHm + heat ⟶ bCO2 + cCO + fossil fuel oil + tar + dH2O
This entry is adapted from the peer-reviewed paper 10.3390/atmos14040655
References
- Di Gianfilippo, M.; Costa, G.; Pantini, S.; Allegrini, E.; Lombardi, F.; Astrup, T.F. LCA of management strategies for RDF incineration and gasification bottom ash based on experimental leaching data. Waste Manag. 2016, 47, 285–298.
- Jardine, C.; Hrudey, S.; Shortreed, J.; Craig, L.; Krewski, D.; Furgal, C.; McColl, S. Risk Management Frameworks for Human Health and Environmental Risks. J. Toxicol. Environ. Health B Crit. Rev. 2003, 6, 569–720.
- Ćetković, J.; Lakić, S.; Živković, A.; Žarković, M.; Vujadinović, R. Economic analysis of measures for GHG emission reduction. Sustainability 2021, 13, 1712.
- Lebreton, L.; Andrady, A. Future scenarios of global plastic waste generation and disposal. Palgrave Commun. 2019, 5, 6.
- Sundarakannan, R.; Arumugaprabu, V.; Manikandan, V.; Vigneshwaran, S. Mechanical property analysis of biochar derived from cashew nut shell waste reinforced polymer matrix. Mater. Res. Express 2019, 6, 125349.
- Yang, R.X.; Jan, K.; Chen, C.T.; Chen, W.T.; Wu, K.C.W. Thermochemical Conversion of Plastic Waste into Fuels, Chemicals, and Value-Added Materials: A Critical Review and Outlooks. ChemSusChem 2022, 15, e202200171.
- Farulla, G.A.; Cellura, M.; Guarino, F.; Ferraro, M. A review of thermochemical energy storage systems for power grid support. Appl. Sci. 2020, 10, 3142.
- Kirtania, K. Thermochemical conversion processes for waste biorefinery. In Waste Biorefinery: Potential and Perspectives; Elsevier: Amsterdam, The Netherlands, 2018.
- Zhang, J.; Zhang, X. The thermochemical conversion of biomass into biofuels. In Biomass, Biopolymer-Based Materials, and Bioenergy: Construction, Biomedical, and Other Industrial Applications; Woodhead Publishing: Sawston, UK, 2019.
- Chiaramonti, D.; Prussi, M.; Buffi, M.; Casini, D.; Rizzo, A.M. Thermochemical Conversion of Microalgae: Challenges and Opportunities. Energy Procedia 2015, 75, 819–826.
- Tan, R.R.; Aviso, K.B.; Foo, D.C.Y. Carbon emissions pinch analysis of economic systems. J. Clean. Prod. 2018, 182, 863–871.
- Priya, G.S.K.; Bandyopadhyay, S. Multiple objectives Pinch Analysis. Resour. Conserv. Recycl. 2017, 119, 128–141.
- Pacheco-López, A.; Lechtenberg, F.; Somoza-Tornos, A.; Graells, M.; Espuña, A. Economic and Environmental Assessment of Plastic Waste Pyrolysis Products and Biofuels as Substitutes for Fossil-Based Fuels. Front. Energy Res. 2021, 9, 676233.
- Li, J.; Burra, K.G.; Wang, Z.; Liu, X.; Kerdsuwan, S.; Gupta, A.K. Energy recovery from composite acetate polymer-biomass wastes via pyrolysis and CO2-assisted gasification. J. Energy Resour. Technol. Trans. ASME 2021, 143, 042305.
- Gamisch, B.; Gaderer, M.; Dawoud, B. On the development of thermochemical hydrogen storage: An experimental study of the kinetics of the redox reactions under different operating conditions. Appl. Sci. 2021, 11, 1623.
- Gabbar, H.A.; Aboughaly, M.; Damideh, V.; Hassen, I. RF-ICP thermal plasma for thermoplastic waste pyrolysis process with high conversion yield and tar elimination. Processes 2020, 8, 281.
- Gupta, A.; Armatis, P.D.; Sabharwall, P.; Fronk, B.M.; Utgikar, V. Kinetics of Ca(OH)2 decomposition in pure Ca(OH)2 and Ca(OH)2-CaTiO3 composite pellets for application in thermochemical energy storage system. Chem. Eng. Sci. 2021, 246, 116986.
- Monni, S. From landfilling to waste incineration: Implications on GHG emissions of different actors. Int. J. Greenh. Gas Control 2012, 8, 82–89.
- Campuzano, F.; Brown, R.C.; Martínez, J.D. Auger reactors for pyrolysis of biomass and wastes. Renew. Sustain. Energy Rev. 2019, 102, 372–409.
- Jagtap, A.; Kalbande, S.R. Investigation on pyrolysis kinetics and thermodynamic parameters of soybean straw: A comparative study using model-free methods. Biomass Convers. Biorefinery 2022.
- Risthaus, K.; Bürger, I.; Lutz, M.; Funayama, S.; Kato, Y.; Linder, M.; Schmidt, M. Experimental and Numerical Investigation of the Dehydration of Ca(OH)2 at Low Steam Pressures. Processes 2022, 10, 325.
- EVenturini, E.; Vassura, I.; Passarini, F.; Bernardi, E.; Ciacci, L.; Ferroni, L. The environmental impact of a municipal solid waste incinerator: 15 years of monitoring. WIT Trans. Ecol. Environ. 2014, 180, 305–316.
- Mao, Z.; Demirgian, J.C. Development of calibration standards for fourier transform infrared spectrometer in continuous monitoring of incinerator emissions. Waste Manag. 1995, 15, 233–241.
- Gabbar, H.A.; Aboughaly, M.; Ayoub, N. Comparative study of MSW heat treatment processes and electricity generation. J. Energy Inst. 2018, 91, 481–488.
This entry is offline, you can click here to edit this entry!
