Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cell Biology
Spheroids and organoids are important novel players in medical and life science research. They are gradually replacing two-dimensional (2D) cell cultures. Indeed, three-dimensional (3D) cultures are closer to the in vivo reality and open promising perspectives for academic research, drug screening, and personalized medicine. A large variety of cells and tissues, including tumor cells, can be the starting material for the generation of 3D cultures, including primary tissues, stem cells, or cell lines.
- 3D cell culture
- tissue engineering
- personalized medicine
1. Introduction
Cancer remains one of the leading causes of death worldwide in the 21st century. Despite intensive progress made in the identification of molecular mechanisms of tumor progression and resistance, as well as in the generation of targeted treatments, many patients are still not cured. With the development and improvement of cell culture techniques, it is to date possible to generate tumor cell cultures in individualized 3D, better mimicking the structure and function of tumors in vivo. These 3D cultures are increasingly being used in cancer research and are particularly useful for three main applications: (i) understanding the pathophysiology of cancer progression and resistance [1,2,3]; (ii) in vitro screening of anti-cancer treatments [4]; and (iii) reproducing in vitro the specificity of one patient’s tumor, to allow a personalized screening of the most effective treatments [5]. Spheroids and organoids are both descriptions used to characterize these cultures. Spheroids are simpler 3D structures that are formed by cells that aggregate together in a spherical shape. They are typically composed of a single-cell type and are used to study cell-to-cell interactions and cell-to-matrix interactions. Spheroids can be generated from a wide variety of cell types, including primary cells, cancer cell lines, and cancer stem cells. Organoids, on the other hand, are more complex structures that replicate the natural tumor architecture and function [6,7]. They are typically composed of multiple cell types, including the tumor microenvironment (TME) [8,9]. The TME is a key factor of tumor aggressiveness and resistance, notably by participating in tumor angiogenesis and immune escape. The ideal 3D tumor culture should preserve not only the molecular signature of the original tumor, but also of its specific TME. This is particularly important in the area of onco-immunology as the TME is involved in the interactions between cancer cells and the host’s immune cells. Cancer organoids including the immune microenvironment can be used to study the effects of various immunotherapies such as checkpoint inhibitors, cancer vaccines, and chimeric antigen receptor (CAR)-T cell therapy [2,10,11].
A key limitation of current 3D methods is the instability of the TME, notably the immune TME, in culture. For example, tumor-infiltrating leucocytes (TILs) are difficult to keep in culture without the addition of specific cytokines. Tumor-infiltrating fibroblasts, on the other hand, are easily expanded in culture with the appropriate medium. To solve these limitations, the addition of non-autologous stromal/immune cells to 3D culture is unfortunately not optimized. In addition to the immune allogenic reaction that would occur in this setting, notably with T cells, the TME is specific to each patient and would not be realistically reproduced in this setting. Thus, the development of new technologies that allow the maintenance of the original TME and its specific composition as close as possible to the original tumor is currently a key challenge. Solving this challenge will notably ensure the concrete development of personalized medicine against cancer in many applications.
2. Current Source to Generate and Culture In Vitro Tumor Spheroids and Organoids
Spheroids and organoids can be generated from different cell and tissue sources, including primary cells/biopsies [12,13,14], cancer stem cell lines, and tumor cell lines [15,16] (Figure 1). As each of these sources has its own advantages and limitations, researchers must carefully consider which source and method is the most appropriate for a specific application.
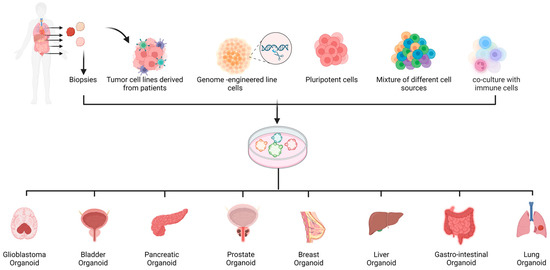
Figure 1. Summary of the procedures used to establish cancer spheroids and organoids. Patients’ primary cells, CRISPR/Cas9 engineered cells, pluripotent stem cells, and a mixture of different cell types and co-culture with immune cells can be used to establish organoids.
2.1. Spheroids from Tumor Cell Lines
Cancer cell lines are isolated from a patient’s tumor and selected for their growth in culture. These cell lines can be propagated indefinitely in culture, which allows for the generation of large numbers of spheroids for experimental purposes. One of the main advantages of using cancer cell lines to generate spheroids [17,18] is that they are readily available and can be purchased from various sources, such as the American Type Culture Collection (ATCC). This makes them easily accessible to researchers. The generated spheroids can be used to study the effects of drugs acting directly on cancer cells [19,20]. Very recently, Husain Yar Khan et al. tested the combination of a new anti-cancer drug (CPI-613) with radiation using a spheroid model of pancreatic cancer (MiaPaCa-2 and Panc-1 cell lines) [21]. Their results show that a combination of radiation with CPI-613 significantly inhibits pancreatic cancer cell growth compared with radiation alone. Moreover, in the context of drug testing in cancer, Sang-Eun Yeon et al. previously showed that Panc-1 spheroids may represent an effective 3D model for anti-pancreatic cancer drug screening [22]. In the context of lung cancer, A. Zuchowska et al. presented a protocol for the generation of spheroids from malignant (A549 cell line) and non-malignant cells with high viability, suitable for drug cytotoxicity studies [23]. In 2018, E. O. Mosaad et al. published a study using a new 3D prostate cancer spheroid platform to perform high-throughput drug screening [24]. Their results demonstrated that spheroids, in comparison to 2D monolayers, are not hypersensitive to chemotherapy, providing a superior model for the evaluation of single and sequential drug treatment. Bladder cancer spheroids were also generated from the RT4-cell line, as a model of drug screening and showed that the obtained spheroids are suitable for drug screening/cytotoxicity assays [25]. In addition to drug testing, spheroids from cell lines are also used in studies dedicated to the investigation of drug resistance [26]. Fan et al. demonstrated that spheroids formed from lung (A549) and pancreatic (PANC1) adenocarcinoma cell lines showed a higher resistance to anti-cancer selenite than cells in 2D [27]. Similarly, in the context of the study of anticancer drug resistance, a German team demonstrated the proof of concept of a high-throughput device allowing the culture, screening, and handling of pancreatic and colorectal spheroids [28]. Renal cell carcinoma spheroids were also used to describe and study the profile of stem cell-like cancer cells, which can also be responsible for metastatic spread and drug resistance [29]. Exploiting a high-throughput automated platform for spheroid culture using a polydimethylsiloxane (PDMS)-based hanging drop array (PDMS-HDA), a study showed that spheroids from several cancer cell lines such as breast, prostate, and colorectal cancer can be generated and show different levels of sensitivity to chemotherapeutic drugs and radiation as compared to 2D cultures [20]. However, there are common limitations in methods using cancer cell lines to generate spheroids [30]. Cancer cell lines are not representative of all patients, since each patient’s molecular signature is unique [31,32]. Moreover, the cancer cell composition is heterogeneous in vivo and it is expected that only some clones with a selective advantage will grow. Furthermore, cancer cell lines can acquire genetic and epigenetic changes over time, which may not reflect the original tumor cell characteristics, and therefore affect the results obtained from the spheroid model. Additionally, cancer cell lines do not represent the TME.
2.2. Organoids Derived from Primary Cells and Patients’ Biopsies
One alternative to cell line-derived spheroids is the generation of organoids from primary tissues such as patient-derived biopsies. Such 3D structures are known as Patient-Derived Organoids (PDOs). PDOs have been successfully generated from several cancer types such as breast cancer [33,34,35,36], lung cancer [37,38], gastro-intestinal cancer [39], gastroesophageal cancer [40], pancreatic cancer [41], ovarian cancer [42], prostate cancer [43], glioblastoma [44], liver cancer [45], colorectal cancer [46], retinoblastoma [47], and also bladder cancer [48]. The patient’s tumor sample may be a solid surgical resection material [33], punch or fine-needle aspiration biopsy [39,41,49,50,51], or biological fluid biopsy [42,51,52,53]. The current process of generating organoids from primary biopsies typically involves the resection of a small part of the tumor, which is cut into smaller pieces, and then mechanically and/or enzymatically dissociated for culture under conditions that promote self-organization into a 3D structure. The culture conditions such as the choice of media and method of organoid generation depend on the type of tumor. These conditions often include the use of specific growth factors and extracellular matrix components that are important for maintaining the proper cellular organization and function. The medium commonly used in many studies is DMEM/F12 medium, due to its richness in nutritional factors and because it is suitable for clonal culture. The latter or another medium is supplemented with small molecules, amino acids, cytokines, growth factors, and other supplements depending on the application and the cancer type [54]. One of the main advantages of using primary biopsies is a more accurate representation of the patient’s specificity, including the molecular signature [47]. PDOs have gained popularity as an effective and rapid 3D tool that better reproduces many tumor features, including the specific genetic and molecular diversity of the original host, hypoxia, nutrient diffusion, and metabolism [55]. PDOs that can retain host-derived TME are a valuable candidate for holistic approaches to 3D immune-oncology TME modeling. However, these models are prohibited by immune cells rapidly losing viability before studies. In a recent study, Yawei Hu et al. described a new and rapid protocol for PDO generation and prediction of drug response [37]. However, obtaining primary cells can be challenging and often require a surgical procedure. Many primary cells of the biopsy have a limited lifespan in culture and are not able to be treated by enzymes and die rapidly, generating a 3D structure in which some cells are missing, notably cells from the TME. For example, in lung cancer, the generation of PDO has, on average, a success rate of only 40%. The failure of PDO generation was associated with the quality of the sample biopsy and also the collection technique [37]. Additionally, variations in growth depend on the source of the biopsy, which can affect the outcome of the studies performed. Another limitation of PDO is the spatial heterogeneity of the tumor of origin; the biopsy may not fully capture the complexity and diversity of the tumor because the sample patient biopsy is often limited [56,57,58,59,60]. Then, the obtained PDO can represent the molecular signature and/or the TME of only a fraction of the original tumor. Sometimes, researchers may have to generate multiple PDOs from different regions of the patient’s tumor in order to study the effects of drugs and other therapies on the entire tumor, which is not easily feasible. Tumor composition and molecular signature vary not only within a patient, but also between patients. If this heterogeneity is exploited for personalized medicine, researchers may need to generate PDOs from multiple patients if their goal is to study the global effects of drugs and other therapies on different types of cancer. In practice, it can be technically, ethically, and financially difficult for researchers to obtain multiple samples from several patients. Therefore, exploiting tissue or cell banks to generate PDO can help to address the problem of patient-to-patient variability [57,58]. Biobanks exist for PDO generation [61] with material from breast cancer biopsies [34,35], pancreatic cancer [62], and colorectal cancer biopsies [63,64,65]. In the context of breast cancer, Norman Sachs et al. generated, in 2018, more than 100 primary and metastatic organoids [35,66]. More recently in 2022, Dan Shu et al. generated a new organoid bank from 17 patients [34]. Biobanks can be used to validate the results obtained from organoids generated from a smaller number of patients. However, biobanks can also have their own limitations [67]. Samples are usually collected at a specific time point, and may not reflect the temporal changes that occur in a patient. Additionally, the samples may not be stored or processed in a standardized manner, which can affect the quality and composition of the samples and make it difficult to compare data.
2.3. Spheroids and Organoids from Genetically Modified Cells
Cancer originates from different and variable genetic and epigenetic aberrations. This variability is recorded in the Cancer Genome Atlas, which describes more than 15,000 tumors [68]. Recently, an important effort has been made to identify the different genetic and epigenetic networks responsible for the cancer development [69]. Genetically modified cells can be used to generate organoids by culturing them in a 3D environment that promotes self-organization. Genetically modified cells to generate cancer organoids include the ability to study specific genetic pathways and the effects of specific mutations that are found in a patient. This can provide a deeper understanding of how genetic changes contribute to the development and progression of cancer and can be used to develop new targeted therapies. Meanwhile, cluster regularly interspaced short palindromic repeats-associated protein 9 (CRISPR/Cas9) technology has revolutionized genome editing and is applicable to the organoid field. CRISPR/Cas9-based gene modification allows the engineering of organoid models of cancer through the introduction of any combination of cancer gene alterations to normal organoids, including knock in (KI) or knockout (KO) of oncogenes or tumor suppressor genes (TS), and gene repression or activation. Multiplex editing by lentivirus or plasmids has been successfully tried [70,71,72,73,74,75,76]. Remarkable results have been achieved in this field. Artegiani et al. introduced BAP1 loss-of-function by CRISPR/Cas9 in normal human cholangiocyte organoids. They observed that BPA1 mutant organoids lost their organization and polarity and cells became more motile and fused with other organoids [77]. These features recapitulated the hallmarks of cancers. Interestingly, after restoring the catalytic activity of BAP1 in the nucleus, they observed a reversion of organoid morphology and molecular alterations to a level similar to WT organoids. CRISPR/Cas9 genome editing was also used in two independent studies in order to model multistep tumorigenesis in normal human intestinal organoids. Matano et al. generated intestinal organoids from normal human intestinal epithelium harboring mutations in the tumors suppressors genes APC, SMAD4, and TP53 and in the oncogenes KRAS and PIK3CA (five hit APC, KRAS, SMAD4, TP53, and PIK3CA (AKSTP)). They demonstrated that organoids carrying these mutations grow independently of all niche factor supplementations and formed tumors after implantation into immunocompromised NOG mice [78]. The same approach was used by Drost et al. to target APC, KRAS, TP53, and SMAD4 genes that designed AKPS in human small intestine and colon organoids. In their study, the authors demonstrated that the quadruple mutant organoids grew and were able to form invasive carcinomas upon subcutaneous xenotransplantation [79]. The approach of genetically engineered-based tumorigenesis has been also extended to normal primary human gastric organoids coupled with gene–drug interaction screens. CRISPR/Cas9-mediated ARID1A/TP53 dual KO organoids mirror several clinical–pathologic features of ARID1A-mutant gastric cancer. A high throughput drug screening revealed that ARID1A deficient gastric organoids were uniquely sensitive to a small molecule inhibitor of BIRC5/surviving [80]. A novel model of CRSIPR-Cas9-engineered TP53-CDKN2A dual KO human normal gastroesophageal junction (GEJ) organoids was generated for the first time. TP53-CDKN2A KO in GEJ induced morphological dysplasia as well as pro-neoplastic features in vitro and in vivo [81]. Takeda Haruna et al. used genetically defined benign tumor-derived organoids carrying two frequent gene mutations (APC and KRAS mutations; AK organoids), which mainly contribute to the disease progress in the early stage of colorectal cancer (CRC) [82]. They demonstrated that AK organoids recapitulate human CRC when transplanted into mice. In an alternative study published in 2016, Verissiomo CS and colleagues tested EGFR and MEK inhibitors on a large panel of CRC organoid lines in order to determine the effect of RAS-mutation status on the sensitivity to these drugs [83]. They demonstrated that the introduction of a KRAS G12D mutation by CRISPR/Cas9 resulted in a loss of drug sensitivity compared to WT. Recently, Neel’s group generated multiple high-grade serous tubo-ovarian (HGSC) cancers by engineering mouse fallopian tube epithelial organoids using lentiviral gene transduction and CRISPR/Cas9 mutagenesis [84]. HGSC models exhibit mutational combinations seen in patients and present several expected but other unanticipated sensitivities to small molecule drugs. In another report, ovarian cancer 3D spheroids were subject to genome editing using CRISPR/Cas9 to inactivate TIMP-2. These modified 3D spheroids exhibited low MMP-2 expression and high MMP-14, TWIST1, and SNAIL expression, enhanced proliferation, migration, and invasion. TIMP-2 KO spheroids were resistant to paclitaxel and formed long sheath-like cell aggregates, which showed enhanced proliferation and expression of the invasion marker KRT14 [85]. Lastly, a study suggested that customized therapy targeting ALDH1 could reduce resistance to chemotherapy and improve the survival rate of ovarian cancer. Consistent with this, ALDH1 inhibition by CRISPR/Cas9 effectively blocked the proliferation and survival of OC spheroids [86]. To summarize, CRISPR/Cas9-generated organoid models can recapitulate the molecular and pathohistological characteristics of human diseases and especially the multistep tumorigenesis from normal cells to malignant cells. Although CRSPR/Cas9 has simplified genetic engineering, there is a considerable margin of error during this process. In order to improve this, a new genetic tool for targeting specific genes in human organoids called CRISPR-Cas9-mediated homology-independent organoid transgenesis (CRISPR-HOT) was pioneered by Artegiani et colleagues. This technique was applied to fluorescently tag and consists of visualizing subcellular structural molecules for rare intestinal subset cells by generating reporters. This technique can be applied to study cell fate, differentiation, and disease development and can be used to visualize any type of gene or cell. This CRISPR-HOT was tried in liver organoids for knock in of cadherin and beta-tubulin genes to label the hepatocyte membrane and mitotic spindle, respectively, for the monitoring of hepatocyte division [87]. Finally, there are also limitations to using genetically modified cells to generate cancer organoids. The genetic modification process itself can introduce further complexity and variability to the organoids, making it harder to draw conclusions from the results obtained.
2.4. Cancer Organoids from Pluripotent Stem Cells
Cancer organoids can be generated from human Pluripotent Stem Cells (hPSCs), which can be embryonic stem cells or induced pluripotent stem cells (iSCs) [88]. The main advantage of using hPSCs is that they can be genetically modified to model specific organs and diseases, including cancer. As previously described, the CRISPR/Cas9 technology can also be applied to introduce specific mutations that are found in human tumors. These modified cells can then be differentiated into organoids that mimic the cellular and molecular features of the corresponding tumor type. Genetically modified organoids from pluripotent stem cells are well suited to investigate cancer development and progression, as well as to be a response to treatment. Brain tumors are among the deadliest and most aggressive cancers worldwide. In a study published in 2018, Shan Bian et al. used genetically modified hPSCs to generate brain cancer organoids [89]. The researchers introduced the mutation into the cells after the first neural induction step by transposon-and CRISPR/Cas9-mediated mutagenesis. These organoids allowed the exploration of the underlying mechanisms of tumor progression and the evaluation of the efficacy of therapies directed at specific genetic mutations. The study also demonstrated that these models are superior to traditional brain tumor spheres and 2D glioblastoma cell cultures as they allow interactions between tumoral and non-tumoral cells within the same system to be revealed. Similarly, in the field of glioblastoma, a study led by Junko Ogawa and colleagues established a cancer model of glioma in human brain organoids for investigating cancer progression, specifically the invasion phase [90]. To generate this model, the authors applied CRISPR/Cas9 technology to integrate an HRasG12V-IRES-tdTomato construct into the TP53 gene by homologous recombination in the H9 hPSC line. Interestingly, the mutant cells quickly became invasive and destroyed the surrounding organoid structures. The invasive nature of these tumor cells was further supported by transplanting the cell into immune-deficient animals. The cells generated by the organoids displayed gene expression profiles that were consistent with human glioblastoma, further illustrating the potential of using organoids as a platform to replicate key features of malignancy. More recently, Markus Breunig et al. developed a pancreatic duct-like organoid (PDLO) model from human pluripotent stem cells to study pancreatic cancer formation from a genetically defined background [91]. They developed a model of pancreatic carcinogenesis by inducing the expression of oncogenes GNAS and KRAS using piggyBac transposon-based vectors combined, or not, with CDKN2A KO by CRISPR/Cas9. Indeed, after PDLO transplantation in mice, they showed that PDLO expressing GNAS formed large oncogenic cystic structures, whereas KRAS alone, induced a diverse range of abnormal growths. However, when KRAS was combined with the loss of CDKN2A, it led to the development of malignant and dedifferentiated pancreatic ductal adenocarcinomas. These results highlighted the possibility to use this PDLO to model the genetic changes that lead to pancreatic cancer formation. Another study in the same field showed that GNAS oncogene expression induces cystic growth more efficiently in ductal organoids than in acinar organoids, while KRAS was more effective in modeling cancer in vivo when expressed in acinar organoids compared to ductal organoids derived from pluripotent stem cells [92]. Using also the activation of KRAS, Antonella F.M. Dost et al. developed an organoid system from human iPSC-derived lung epithelial cells to model early-stage lung adenocarcinoma, illustrating another time that the potential of cancer organoids is derived from hPSCs [93]. The study demonstrated that alveolar epithelial progenitor cells that expressed oncogenic KRAS showed a decrease in the expression of maturation genes. This research provides a comprehensive dataset that differentiates normal epithelial progenitor cells from those in early-stage lung cancer, making it easier to identify targets for KRAS-driven tumors. While organoids generated from PSCs have a great potential to model diseases, to identify new therapeutic targets and to test the efficacy of different drugs on the tumors, there are some technical and ethical challenges that need to be considered. For example, there is a risk of contamination with undifferentiated stem cells, and the organoids may not fully replicate the complex cellular interactions of a living organism; therefore, they may not fully mimic the disease in vivo. Moreover, there are some ethical concerns associated with the use of embryonic stem cells. Finally, the capacity to genetically modify hPSCs has enormous potential for cancer modelization through cancer organoids. However, while it has been relatively easy to edit immortalized human tumor cell lines [94], it is not as easy with hPSCs [95,96,97].
2.5. Organoids Made from Several Cell Sources
Organoids can be generated from a mixture of different cell sources, including primary cells, endothelial cells, stromal cells, or tumor cell lines. Combining different cell types in a mixture can create an environment that closely mimics the complexity of the in vivo TME. Yeonhwa Song and colleagues developed an organoid model using several cell types such as hepatocellular carcinoma cells, fibroblasts (WI38 cell line), hepatic stellate cells (LX2 line), and the endothelial primary cell line HUVEC to establish a liver fibrosis model [98]. The aim of this study was to identify potential mechanisms and inhibitors of liver fibrosis, suggesting that anti-fibrosis drugs may improve tissue permeability to support the delivery and efficacy of anti-cancer drugs. This model has the potential to offer an efficient strategy to identify new drugs and targets in an accurate organoid model close to the in vivo situation. Similarly, to recapitulate bone marrow in both normal and tumoral conditions, many different studies have been performed [99,100]. Recent studies have developed bone marrow organoids (BMOs) [101,102,103]. Recently, we developed a BMO model that mimics several structural and cellular features of native bone marrow (BM) [103]. These BMOs were formed from Mesenchymal Stromal Cells (MSCs) and endothelial cells, modeling the migration and integration of leukemic cells within the BM, highlighting the potential of this model as an easily accessible and scalable preclinical model for evaluating the efficacy of potential drugs in personalized medicine. Another BMO model has been developed recently, using hPSCs differentiated into mesenchymal, endothelial, and hematopoietic lineages. It recapitulated the stroma, sinusoids that form lumens, and myeloid cells, including proplatelet-forming megakaryocytes [102]. This model can also sustain primary cells from patients of myeloid and lymphoid blood cancers within the context of their microenvironment and represents a crucial ex vivo tool for evaluating new therapeutics. One advantage of using a mixture of cell sources to generate organoids is that it allows researchers to study the interactions between different cell types in the TME and their impact. For example, H. Zhao et al. investigated the co-culture of tumor-infiltrating fibroblasts with oral squamous cell carcinoma organoids. They showed that the co-culture with fibroblast promoted the stemness properties of the primary carcinoma [104]. However, generating organoids from a mixture of cell sources can be technically challenging [105], especially if the cell types that need to be mixed have different culture requirements or different growth rates. Additionally, the organoids may not fully replicate the complex cellular interactions of a living organism, and there can be variations depending on the cell source used and the method of preparation.
2.6. Organoids Including the TME
Cancer organoids have a limitation in their application when studying the impact of the surrounding TME, as they often lack the representation of immune cells [106,107]. Recent studies have therefore focused on the development of cancer organoids that include immune cells. These models have been used to study the interactions between cancer cells and the immune system, and to test immunotherapies. By including immune cells and components, these organoids better mimic the patient’s TME and can provide valuable insights into cancer biology including the mechanisms of immune evasion and resistance [108]. They can also be used to identify new targets for immunotherapy and to evaluate the efficacy of new drugs. The types of immune cells that should be included in cancer organoids depend on the research question and the type of cancer being studied. Some common immune cells included in cancer organoids include T cells (cytotoxic or regulatory), NK cells, macrophages, dendritic cells (DCs), myeloid-derived suppressor cells (MDSCs), or neutrophils. The immune cells must be derived from the same patient to study the patient-specific immune response and prevent allogeneic responses. A study presented a method successfully preserving several endogenous immune cell types including T cells, macrophages, B, and NK cells, in PDOs [64]. These organoids were derived from more than 100 human tumor biopsies of skin, kidney, and lung cancers and were used to study the effects of drugs that inhibit immune checkpoint proteins such as PD-1 and PD-L1 [64]. Another study performed a co-culture of two different human lung cancer cell lines (A549 and Calu-6) alone or together with fibroblasts and peripheral blood mononuclear cells (PBMCs) [109]. The authors showed that when the cancer cells were cultivated with fibroblasts, the infiltration capacity of cytotoxic T lymphocytes was increased, demonstrating that (i) immune cells could be added to the cancer 3D model, and (ii) the TME significantly impacted immune cell infiltration and activation. In addition, the inclusion of fibroblasts in cancer organoids has been shown to lead to a change in the type of T-cells present, with a greater proportion of activated ones [110]. There are still a number of limitations to the general use of organoids that include immune cells [61]. One of the main limitations is the complexity of the TME and the difficulty to define or keep its original composition in culture. Moreover, the use of organoids to study cancer immunotherapies is still relatively new, and there is still much to be learned about how these models can be used to predict responses to therapy in patients. Then, there is always the concern that the organoids may not fully represent the in vivo biology of the tumor and its immune system. Thus, methods that will ensure the complete maintenance of the original TME will be the better options.
This entry is adapted from the peer-reviewed paper 10.3390/cells12071001
This entry is offline, you can click here to edit this entry!
