The entry hereby provides basic information about COVID-19 disease and its causative agent SARS-CoV-2 virus. Also, graphical representation of all the vaccine candidates under preclinical and clinical trials based on different technical platforms is shown. Importantly, immune responses generated during natural COVID-19 infection in recovered patients are discussed with their implications in vaccine development.
- COVID-19
- Vaccine platforms
- recovered patients
- neutralizing antibody titers
- T-cell responses
1. Introduction
Coronavirus disease 2019 (COVID-19) pandemic has resulted in nearly 60 million infections, claiming around 1.5 million human lives globally (https://coronavirus.jhu.edu). COVID-19 is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a beta-coronavirus that emerged from bats and then transferred to humans through intermediate hosts [1,2,3]. SARS-CoV-2 transmits from human to human via respiratory droplets leading to a respiratory tract infection that can progress to severe pneumonia, multiple organ involvement, and fatal outcomes [4,5,6]. The SARS-CoV-2 infection can be asymptomatic or symptomatic with mild to life-threatening symptoms [6,7,8,9,10]. Irrespective of the severity of symptoms, an infected individual is very likely to spread the infection and especially pose a greater risk to the vulnerable population [11,12,13].
A vaccine is urgently needed to control the current exploding global pandemic of COVID-19 and prevent recurrent epidemics. COVID-19 combined with the seasonal Flu epidemic is expected to further aggravate the situation in terms of diagnosis, co-infections, and severity of the disease. An effective vaccine would check on the ongoing health or medical crisis and improve the socio-economic status of the society that has severely been impacted due to the COVID-19 pandemic [14,15]. Additionally, a return to normalcy with no social distancing or masks can be achieved once all are vaccinated and immune to COVID-19. Hence, eradicating SARS-CoV-2 is very much needed through an effective vaccine, especially in the absence of specific licensed drugs to treat COVID-19.
Since SARS-CoV-2 is closely related to severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), and other coronaviruses, vaccine design greatly relies on the existing preventive strategies that have been tested for these viruses at the preclinical or clinical level [16,17,18,19]. While virus-neutralizing antibody responses will always be the hallmark for all anti-viral vaccines, some vaccines have also shown the potential to induce protective T-cell responses [16,20,21,22,23]. It is currently not entirely known as to what will prove to be a correlate of protection against this novel coronavirus.
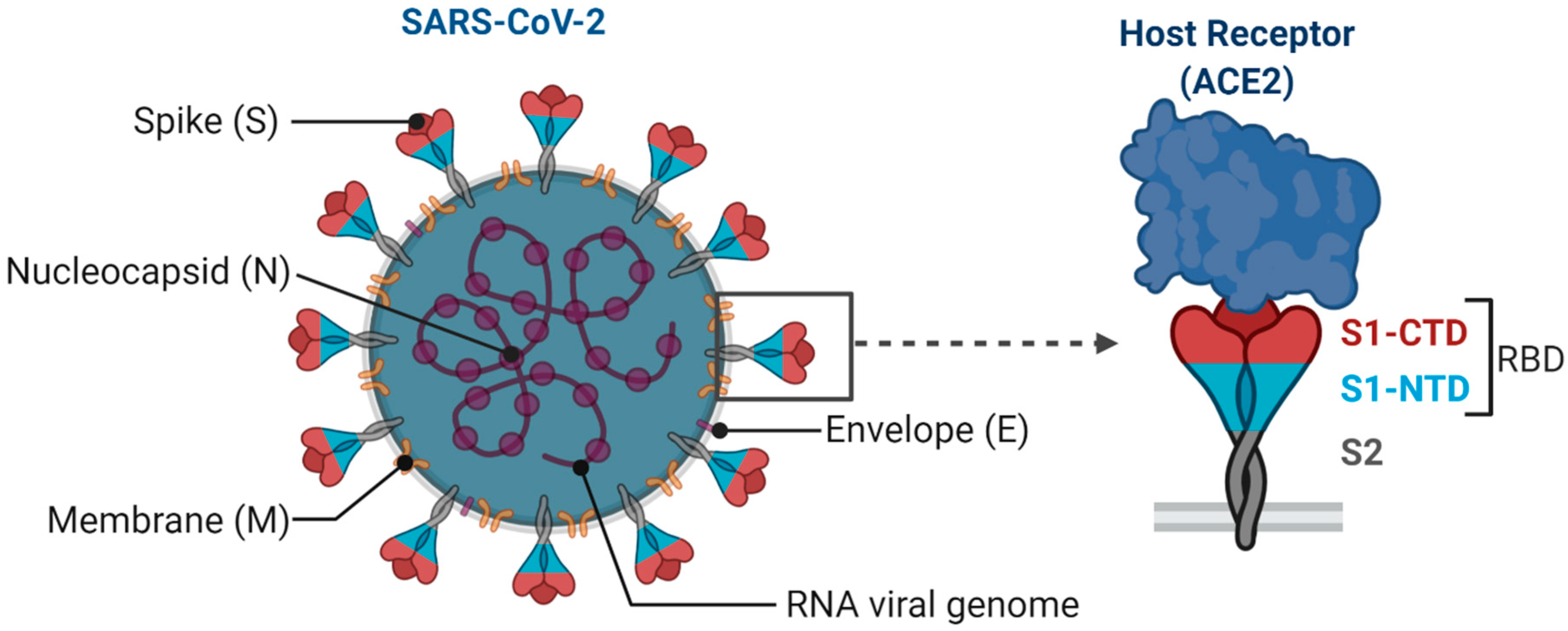
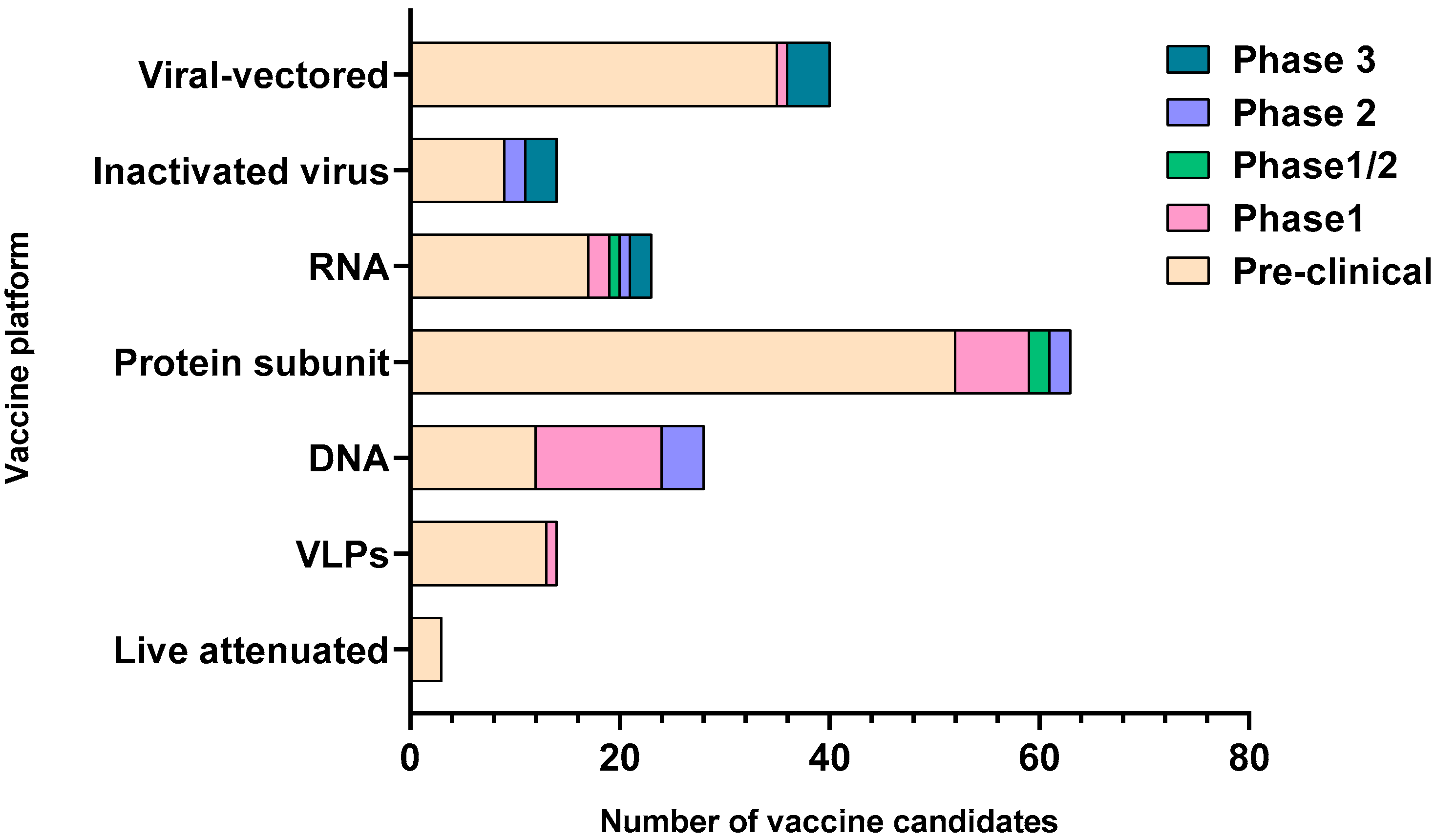
SARS-CoV-2 is an enveloped single-stranded RNA virus. The viral envelope is embedded with spike (S) glycoprotein, the matrix (M) protein, and envelope (E) protein. Encased into this envelope is a positive-sense single-stranded RNA as a viral genome bound to helical nucleocapsid (N) protein (Figure 1) [24,25]. The viral spike protein that mediates entry into the host cell has been identified as one of the preferred vaccine targets. SARS-CoV-2 targets the ACE2 receptor on the host cell via its spike Protein (S), composed of S1 and S2 subunits [24,26]. The S1 subunit contains the receptor-binding domain (RBD) that interacts with the ACE2 receptor, thereby inducing a series of conformational changes facilitating membrane fusion and entry [26]. Due to the critical role played by the spike protein in mediating viral attachment and entry, nearly all SARS-CoV-2 vaccines currently in development are predominantly focused on eliciting protective immune responses targeting the viral spike [21,23,25,26,27,28,29,30]. Although the spike protein is the major component of most vaccines, the technical platform determines how different platforms can modulate the immune responses. Therefore, safety, immunogenicity, and efficacy will majorly depend on the vaccine approach or delivery platforms. In general, vaccine platforms are broadly categorized into six types: live attenuated viral vaccine, inactivated virus vaccine, recombinant viral-vectored vaccines, protein subunit vaccines, virus-like particles (VLPs), and nucleic acid-based (DNA or mRNA) vaccines (https://www.vaccines.gov/basics/types). The relative progress of the vaccine candidates towards different stages of clinical development is shown in Figure 2.
2. Insights from Immune Responses Elicited in the Recovered Patients for Vaccine Development
A more in-depth understanding of the immune responses elicited in recovering COVID-19 patients might provide useful insights into vaccine design. As a hallmark for an effective vaccine, the induction of virus-neutralizing antibody responses is often considered inevitable. With the recent approval of convalescent plasma therapy, the U.S. FDA ignited great interest in its therapeutic potential. However, several independent studies performed at multiple locations globally have shown that nAbs titers in the plasma of mildly symptomatic patients recovering from COVID-19 are highly variable [77,78,79]. A similar study, based on 68 convalescent SARS-CoV-2 patients, by Robbiani et al. showed that on average, the nAb titers remained low in these patients, even undetectable in 18% of them, while only 3% had high nAb titers [80]. Variability in nAbs titers was also reported by Wu et al., based on a cohort study of 175 convalescent COVID-19 patients [81]. Additionally, they observed higher nAb titers in older rather than younger people. Both studies also confirmed the presence of spike and RBD-specific antibodies, titers of which directly correlated with virus neutralization. Interestingly, RBD-specific antibodies were effective even at much lower titers when tested for virus neutralization in in vitro assays.
Furthermore, RBD-specific B-cell precursors were identified to be commonly prevalent in patients based on antibody sequencing data [80,82,83]. Studies have also shown that several epitopes were targeted exclusively in the RBD region for antibody generation in natural infections. The majority of such antibodies proved potent in virus neutralization [80]. Other than spike-directed responses, antibodies targeting the nucleoprotein (NP) of SARS-CoV-2 were also observed with the potential of virus neutralization in the COVID-19 infected patients [84]. However, expected variability in immune responses is due to many factors such as age, sex, geographical location, and prevalent strain of the virus. Considering wide variations in nAbs titers in the convalescent patients and a lack of correlation with the disease and the recovery’s mild outcome, it is difficult to say if vaccine-elicited nAbs would be enough for adequate protection against SARS-CoV-2.
To mount a robust immune response against an invading pathogen, both adaptive and innate arms of the immune system work in conjunction. Though antibodies are traditionally considered necessary molecules of immune defense, their generation relies on effective cross-talk with the T-cells. Griffoni et al. detected SARS-CoV-2-specific CD4+ and CD8+ T-cells in convalescent patients based on predicted T-cell epitopes spanning the whole viral genome. Spike-specific CD4+ T-cell responses were exceptionally prevalent in all infected individuals and were notably correlated with the anti-spike RBD antibody responses [85]. Unlike SARS infections, where T-cell responses were predominantly directed to the viral spike, in SARS-CoV-2 infections, M and N proteins, along with a spike, were targeted to elicit T-cell responses.
Furthermore, CD4+ T-cells responses were directed towards the non-structural antigens such as; nsp4, ORF3s, ORF7a, nsp12, and ORF8 [85]. This suggests that although spike/RBD is a prime vaccine target for all current vaccine development approaches, the inclusion of other structural and non-structural viral antigens might better recapitulate a scenario occurring in the convalescent patients after natural infection. Overall, T-cell immunity has been positively correlated with improved recovery in infected patients, and SARS-CoV-2 infected individuals with severe disease have been shown to undergo T-cell lymphopenia [86]. Some studies have also predicted the occurrence of T-cell exhaustion in COVID-19 patients [87]. Although virus targeted T-cell immune responses might be beneficial to consider for vaccine development, T-cell immunopathologies should be monitored in vaccine recipients, as these undesirable responses have previously been observed for SARS vaccine candidates.
Developing a vaccine that can cross-protect against similar coronaviruses would be an ideal consideration for the future. SARS shares about 80% of the sequence homology with SARS-CoV-2 at the genomic level, with both viruses utilizing the ACE2 receptor for the host cell entry [24,88,89]. While these commonalities have shown cross-reactive responses as observed by many studies, cross-protection could not become evident. This cross-reactivity is majorly attributed to the conserved viral antigenic epitopes and would be worth considering while designing a broadly cross-protective vaccine against related coronaviruses. However, such attempts should be made cautiously as the presence of cross-reactive antibodies has also been previously observed to enhance the infection through antibody-dependent enhancement (ADE) in case of other viral infections, including SARS [18,90]. Thus, despite the urgency of a SARS-CoV-2 vaccine, both the safety and efficacy should be critically evaluated before licensing a vaccine.
This entry is adapted from the peer-reviewed paper 10.3390/vaccines8040649
