Microalgae and cyanobacteria include procaryotic and eucaryotic photosynthetic micro-organisms that produce biomass rich in biomolecules with a high value. Some examples of these biomolecules are proteins, lipids, carbohydrates, pigments, antioxidants, and vitamins. Microalgae are also considered a good source of biofuel feedstock. The microalga-based biorefinery approach should be used to promote the sustainability of biomass generation since microalga biomass production can be performed and integrated into a circular bioeconomy structure. To include an environmentally sustainable approach with microalga cultures, it is necessary to develop alternative ways to produce biomass at a low cost, reducing pollution and improving biomass development. Different strategies are being used to achieve more productivity in cultivation, such as magnets in cultures. Magnetic forces can alter microalga metabolism, and this field of study is promising and innovative, remains an unexplored area.
- microalgae
- magnetic field
- growth rate
- chemical composition
- algal biorefinery
- environmental safety
1. Introduction
2. Microalga Cultures and Magnetic Fields

3. Microalga-Based Biorefinery
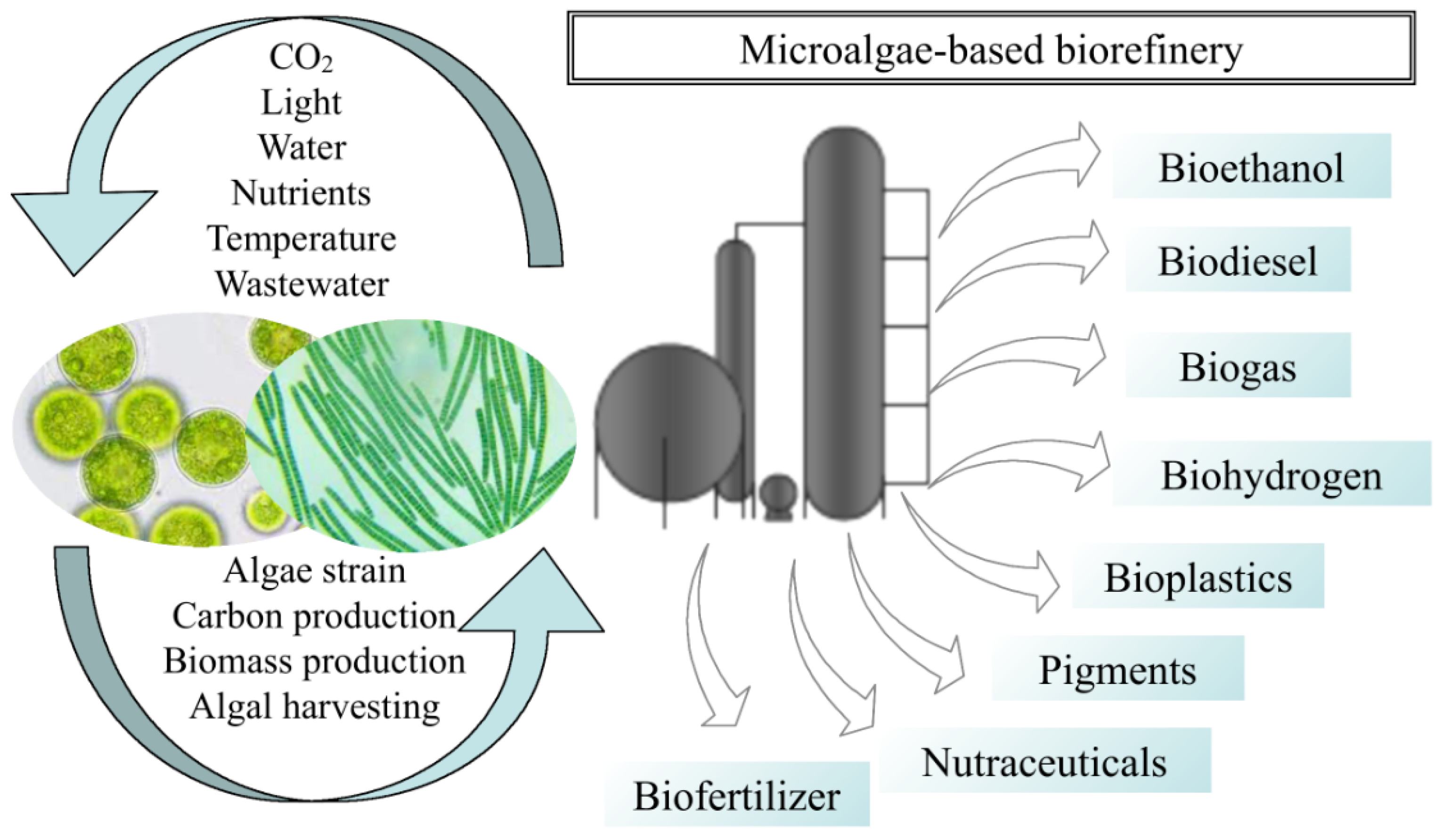
4. Sustainable and Environmentally Friendly Application of Microalgae
This entry is adapted from the peer-reviewed paper 10.3390/su142013291
References
- Chew, K.W.; Yap, J.Y.; Show, P.L.; Suan, N.H.; Juan, J.C.; Ling, T.C.; Lee, D.-J.; Chang, J.-S. Microalgae biorefinery: High value products perspectives. Bioresour. Technol. 2017, 229, 53–62.
- Hunt, R.W.; Zavalin, A.; Bhatnagar, A.; Chinnasamy, S.; Das, K.C. Electromagnetic biostimulation of living cultures for biotechnology, biofuel and bioenergy applications. Int. J. Mol. Sci. 2009, 10, 4515–4558.
- Spanò, N.; Di Paola, D.; Albano, M.; Manganaro, A.; Sanfilippo, M.; D’Iglio, C.; Capillo, G.; Savoca, S. Growth performance and bioremediation potential of Gracilaria gracilis (Steentoft, L.M. Irvine & Farnham, 1995). Int. J. Environ. Stud. 2022, 79, 748–760.
- Tu, R.; Jin, W.; Xi, T.; Yang, Q.; Han, S.-F.; Abomohra, A.E.-F. Effect of static magnetic field on the oxygen production of Scenedesmus obliquus cultivated in municipal wastewater. Water Res. 2015, 86, 132–138.
- Han, S.; Jin, W.; Chen, Y.; Tu, R.; Abomohra, A.E.-F. Enhancement of lipid production of Chlorella pyrenoidosa cultivated in municipal wastewater by magnetic treatment. Appl. Biochem. Biotechnol. 2016, 180, 1043–1055.
- Santos, L.O.; Deamici, K.M.; Menestrino, B.C.; Garda-Buffon, J.; Costa, J.A.V. Magnetic treatment of microalgae for enhanced product formation. World J. Microbiol. Biotechnol. 2017, 33, 169.
- Zieliński, M.; Rusanowska, P.; Dębowski, M.; Hajduk, A. Influence of static magnetic field on sludge properties. Sci. Total Environ. 2018, 625, 738–742.
- Bauer, L.M.; Costa, J.A.V.; da Rosa, A.P.C.; Santos, L.O. Growth stimulation and synthesis of lipids, pigments and antioxidants with magnetic fields in Chlorella kessleri cultivations. Bioresour. Technol. 2017, 244, 1425–1432.
- Serrano, G.; Miranda-Ostojic, C.; Ferrada, P.; Wulff-Zotelle, C.; Maureira, A.; Fuentealba, E.; Gallardo, K.; Zapata, M.; Rivas, M. Response to Static Magnetic Field-Induced Stress in Scenedesmus obliquus and Nannochloropsis gaditana. Mar. Drugs 2021, 19, 527.
- Costa, S.S.; Peres, B.P.; Machado, B.R.; Costa, J.A.V.; Santos, L.O. Increased lipid synthesis in the culture of Chlorella homosphaera with magnetic fields application. Bioresour. Technol. 2020, 315, 123880.
- Deamici, K.M.; Santos, L.O.; Costa, J.A.V. Magnetic field action on outdoor and indoor cultures of Spirulina: Evaluation of growth, medium consumption and protein profile. Bioresour. Technol. 2018, 249, 168–174.
- Small, D.P.; Hüner, N.P.; Wan, W. Effect of static magnetic fields on the growth, photosynthesis and ultrastructure of Chlorella kessleri microalgae. Bioelectromagnetics 2012, 33, 298–308.
- Deamici, K.M.; Costa, J.A.V.; Santos, L.O. Magnetic fields as triggers of microalga growth: Evaluation of its effect on Spirulina sp. Bioresour. Technol. 2016, 220, 62–67.
- Veiga, M.C.; Fontoura, M.M.; de Oliveira, M.G.; Costa, J.A.V.; Santos, L.O. Magnetic fields: Biomass potential of Spirulina sp. for food supplement. Bioprocess Biosyst. Eng. 2020, 43, 1231–1240.
- Deamici, K.M.; Santos, L.O.; Costa, J.A.V. Magnetic field as promoter of growth in outdoor and indoor assays of Chlorella fusca. Bioprocess Biosyst. Eng. 2021, 44, 1453–1460.
- Deamici, K.M.; de Morais, M.G.; Santos, L.O.; Muylaert, K.; Gardarin, C.; Costa, J.A.V.; Laroche, C. Static magnetic fields effects on polysaccharides production by different microalgae strains. Appl. Sci. 2021, 11, 5299.
- Deamici, K.M.; Santos, L.O.; Costa, J.A.V. Use of static magnetic fields to increase CO2 biofixation by the microalga Chlorella fusca. Bioresour. Technol. 2019, 276, 103–109.
- Chu, F.-J.; Wan, T.-J.; Pai, T.-Y.; Lin, H.-W.; Liu, S.-H.; Huang, C.-F. Use of magnetic fields and nitrate concentration to optimize the growth and lipid yield of Nannochloropsis oculata. J. Environ. Manag. 2020, 253, 109680.
- Dębowski, M.; Zieliński, M.; Kisielewska, M.; Hajduk, A. Effect of constant magnetic field on anaerobic digestion of algal biomass. Environ. Technol. 2016, 37, 1656–1663.
- Huo, S.; Chen, X.; Zhu, F.; Zhang, W.; Chen, D.; Jin, N.; Cobb, K.; Cheng, Y.; Wang, L.; Ruan, R. Magnetic field intervention on growth of the filamentous microalgae Tribonema sp. in starch wastewater for algal biomass production and nutrients removal: Influence of ambient temperature and operational strategy. Bioresour. Technol. 2020, 303, 122884.
- Sirisha, K.; Suganya, B.; Sivasubramanian, V.; Bs, V.; Swaminathan, D.; Babu, A.; Meyyappan, N. Studies on the effect of pulsed magnetic field on the productivity of algae grown in dye industry effluent. J. Appl. Biotechnol. Bioeng. 2017, 3, 409–413.
- Sambusiti, C.; Bellucci, M.; Zabaniotou, A.; Beneduce, L.; Monlau, F. Algae as promising feedstocks for fermentative biohydrogen production according to a biorefinery approach: A comprehensive review. Renew. Sustain. Energy Rev. 2015, 44, 20–36.
- Goswami, R.K.; Mehariya, S.; Verma, P.; Lavecchia, R.; Zuorro, A. Microalgae-based biorefineries for sustainable resource recovery from wastewater. J. Water Process Eng. 2021, 40, 101747.
- Jalilian, N.; Najafpour, G.D.; Khajouei, M. Macro and micro algae in pollution control and biofuel production—A review. ChemBioEng Rev. 2020, 7, 18–33.
- Vieira, M.V.; Pastrana, L.M.; Fuciños, P. Microalgae encapsulation systems for food, pharmaceutical and cosmetics applications. Mar. Drugs 2020, 18, 644.
- Santos, L.O.; Silva, P.G.P.; Costa, S.S.; Machado, T.B. Magnetic Field Application to Increase Yield of Microalgal Biomass in Biofuel Production. In Biotechnological Applications of Biomass; IntechOpen: London, UK, 2020.
- Alam, M.A.; Xu, J.-L.; Wang, Z. Microalgae Biotechnology for Food, Health and High Value Products; Springer: Berlin/Heidelberg, Germany, 2020.
- Esteves, A.F.; Soares, O.S.; Vilar, V.J.; Pires, J.C.; Gonçalves, A.L. The effect of light wavelength on CO2 capture, biomass production and nutrient uptake by green microalgae: A step forward on process integration and optimisation. Energies 2020, 13, 333.
- Menegazzo, M.L.; Fonseca, G.G. Biomass recovery and lipid extraction processes for microalgae biofuels production: A review. Renew. Sustain. Energy Rev. 2019, 107, 87–107.
- Debnath, C.; Bandyopadhyay, T.K.; Bhunia, B.; Mishra, U.; Narayanasamy, S.; Muthuraj, M. Microalgae: Sustainable resource of carbohydrates in third-generation biofuel production. Renew. Sustain. Energy Rev. 2021, 150, 111464.
- de Carvalho Silvello, M.A.; Gonçalves, I.S.; Azambuja, S.P.H.; Costa, S.S.; Silva, P.G.P.; Santos, L.O.; Goldbeck, R. Microalgae-based carbohydrates: A green innovative source of bioenergy. Bioresour. Technol. 2022, 344, 126304.
- Daneshvar, E.; Ok, Y.S.; Tavakoli, S.; Sarkar, B.; Shaheen, S.M.; Hong, H.; Luo, Y.; Rinklebe, J.; Song, H.; Bhatnagar, A. Insights into upstream processing of microalgae: A review. Bioresour. Technol. 2021, 329, 124870.
- Berlot, M.; Rehar, T.; Fefer, D.; Berovic, M. The influence of treatment of Saccharomyces cerevisiae inoculum with a magnetic field on subsequent grape must fermentation. Chem. Biochem. Eng. Q. 2013, 27, 423–429.
- Deutmeyer, A.; Raman, R.; Murphy, P.; Pandey, S. Effect of magnetic field on the fermentation kinetics of Saccharomyces cerevisiae. Adv. Biosci. Biotechnol. 2011, 2011, 6857.
- Santos, L.O.; Alegre, R.M.; Garcia-Diego, C.; Cuellar, J. Effects of magnetic fields on biomass and glutathione production by the yeast Saccharomyces cerevisiae. Process Biochem. 2010, 45, 1362–1367.
- da Silva, P.G.P.; Júnior, D.P.; Sala, L.; de Medeiros Burkert, J.F.; Santos, L.O. Magnetic field as a trigger of carotenoid production by Phaffia rhodozyma. Process Biochem. 2020, 98, 131–138.
- Tan, L.; Shao, Y.; Mu, G.; Ning, S.; Shi, S. Enhanced azo dye biodegradation performance and halotolerance of Candida tropicalis SYF-1 by static magnetic field (SMF). Bioresour. Technol. 2020, 295, 122283.
- Zhang, J.; Zhou, K.; Wang, L.; Gao, M. Extremely low-frequency magnetic fields affect pigment production of Monascus purpureus in liquid-state fermentation. Eur. Food Res. Technol. 2014, 238, 157–162.
- Gao, M.; Zhang, J.; Feng, H. Extremely low frequency magnetic field effects on metabolite of Aspergillus niger. Bioelectromagnetics 2011, 32, 73–78.
- Canli, O.; Kurbanoglu, E.B. Application of low magnetic field on inulinase production by Geotrichum candidum under solid state fermentation using leek as substrate. Toxicol. Ind. Health 2012, 28, 894–900.
- Menestrino, B.d.C.; Pintos, T.H.C.; Sala, L.; Costa, J.A.V.; Santos, L.O. Application of static magnetic fields on the mixotrophic culture of Chlorella minutissima for carbohydrate production. Appl. Biochem. Biotechnol. 2020, 192, 822–830.
- Pérez, A.T.E.; Camargo, M.; Rincón, P.C.N.; Marchant, M.A. Key challenges and requirements for sustainable and industrialized biorefinery supply chain design and management: A bibliographic analysis. Renew. Sustain. Energy Rev. 2017, 69, 350–359.
- Pessôa, L.C.; Deamici, K.M.; Pontes, L.A.M.; Druzian, J.I.; de Jesus Assis, D. Technological prospection of microalgae-based biorefinery approach for effluent treatment. Algal Res. 2021, 60, 102504.
- Wang, Y.; Ho, S.-H.; Cheng, C.-L.; Guo, W.-Q.; Nagarajan, D.; Ren, N.-Q.; Lee, D.-J.; Chang, J.-S. Perspectives on the feasibility of using microalgae for industrial wastewater treatment. Bioresour. Technol. 2016, 222, 485–497.
- Ferreira, G.F.; Rios Pinto, L.F.; Carvalho, P.O.; Coelho, M.B.; Eberlin, M.N.; Maciel Filho, R.; Fregolente, L.V. Biomass and lipid characterization of microalgae genera Botryococcus, Chlorella, and Desmodesmus aiming high-value fatty acid production. Biomass Convers. Biorefinery 2021, 11, 1675–1689.
- Suganya, T.; Varman, M.; Masjuki, H.; Renganathan, S. Macroalgae and microalgae as a potential source for commercial applications along with biofuels production: A biorefinery approach. Renew. Sustain. Energy Rev. 2016, 55, 909–941.
- Costa, J.A.V.; Freitas, B.C.B.; Cruz, C.G.; Silveira, J.; Morais, M.G. Potential of microalgae as biopesticides to contribute to sustainable agriculture and environmental development. J. Environ. Sci. Health Part B 2019, 54, 366–375.
- Bhattacharjee, R.; Dey, U. Biofertilizer, a way towards organic agriculture: A review. Afr. J. Microbiol. Res. 2014, 8, 2332–2343.
- Thirumurthy, P.; Mol, I. Microalgae as bio-pesticides for the development of sustainable agriculture. Wide Spectr. 2020, 6, 5–22.
- Costa, J.A.V.; Cassuriaga, A.P.A.; Moraes, L.; de Morais, M.G. Biosynthesis and potential applications of terpenes produced from microalgae. Bioresour. Technol. Rep. 2022, 101166.
- Stirk, W.; Bálint, P.; Tarkowská, D.; Novák, O.; Maróti, G.; Ljung, K.; Turečková, V.; Strnad, M.; Ördög, V.; Van Staden, J. Effect of light on growth and endogenous hormones in Chlorella minutissima (Trebouxiophyceae). Plant Physiol. Biochem. 2014, 79, 66–76.
- GMI (Global Market Insights). Algae Oil Market Size, Regional Outlook, Application Growth Potential, COVID-19 Impact Analysis, Competitive Market Growth & Forecast, 2022–2028; Global Market Insights Inc.: Selbyville, DE, USA, 2022.
- Qari, H.; Rehan, M.; Nizami, A.-S. Key issues in microalgae biofuels: A short review. Energy Procedia 2017, 142, 898–903.
- Dineshbabu, G.; Goswami, G.; Kumar, R.; Sinha, A.; Das, D. Microalgae—Nutritious, sustainable aqua-and animal feed source. J. Funct. Foods 2019, 62, 103545.
- The State of World Fisheries and Aquaculture 2018—Meeting the Sustainable Development Goals; The State of The World series of the Food and Agriculture Organization of the United Nations. Aquaculture; FAO: Rome, Italy, 2018; Volume 35.
- Hunter, M.C.; Smith, R.G.; Schipanski, M.E.; Atwood, L.W.; Mortensen, D.A. Agriculture in 2050: Recalibrating targets for sustainable intensification. Bioscience 2017, 67, 386–391.
- Hasan, M.; Soto, D. Improving Feed Conversion Ratio and Its Impact on Reducing Greenhouse Gas Emissions in Aquaculture; FAO: Rome, Italy, 2017.
