2. The Dynamic Nature of Microtubules
Microtubules are dynamic polymers of α/β-tubulin heterodimers found in all eukaryote cells. These polymers are intrinsically polar, and they stochastically change between phases of growth and shrinkage: a phenomenon known as dynamic instability (see
Figure 1). This MT behavior is associated with the requirement of GTP-tubulin heterodimers for MT polymerization and GTP hydrolysis by β-tubulin after polymerization [
4]. However, the dynamic instability of MTs cannot explain all features of MT dynamics, such as, for example, the dynamics of aged MT. Thus, more recently, MT tip structures have also been implicated in the mechanisms of tubulin polymerization and dynamic instability (for review [
4]). In vivo, MT dynamics depend on the presence of competent tubulin heterodimers, either synthesized de novo or recycled from pre-existing MTs [
5]. To support this process, eukaryotic cells have several molecular chaperones, including the cytosolic chaperonin CCT (cytosolic chaperonin-containing TCP1) and its cochaperone prefoldin [
6,
7], as well as a group of specialized tubulin cofactors (TBCA-E) [
8,
9]. In addition to supporting tubulin folding, tubulin cofactors also aid in the assembly/disassembly of tubulin heterodimers, as well as their degradation (see
Figure 1) [
8,
9,
10,
11]. Therefore, tubulin cofactors are essential for preserving the quality of tubulin pools and the recycling/degradation of tubulin heterodimers in vivo.
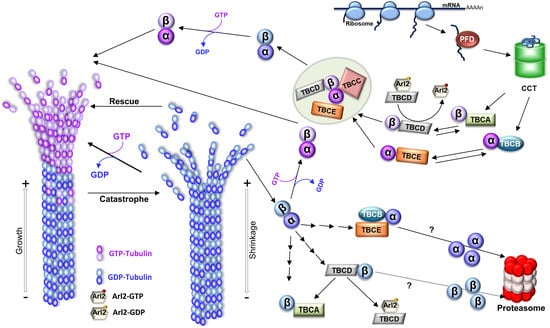
Figure 1. Microtubule structure, dynamics, and tubulin folding and recycling pathways. To originate MTs, tubulin heterodimers interact head-to-tail, arranging in linear protofilaments. The lateral association of the protofilaments, in general, 13 protofilaments, creates the hollow cylindrical structure of the MT with about 25 nm of diameter. Since tubulin heterodimers are oriented inside the MT, this produces a structural polarity; one end of the MT always initiates with α-tubulin, whereas the other terminates with β-tubulin, which is reflected in the dynamic behavior of the polymer with the two ends of a MT displaying different rates of polymerization, a faster-growing end (where β-tubulin is exposed; plus end), and a slower-growing end (where the α-tubulin is ex-posed; minus end). MTs polymerize from soluble tubulin heterodimers bound to guanosine triphosphate (GTP). After incorporation in the polymer, the β-tubulin GTPase activity is activated, GTP is hydrolyzed to guanosine diphosphate (GDP), and the energy from that hydrolysis is assumed to induce conformational changes in tubulin, reducing the stability of the MT lattice (for review [
12]). In growing MTs, most heterodimers in the lattice are bound to GDP. However, whenever a MT maintains a cap of GTP tubulin heterodimers at the tip, the entire polymer will be stabilized (the GTP cap model), and the MT grows. On the contrary, if the tip of the MT accumulates a critical number or density of GDP-tubulins, the MT lattice will become unstable, and the MT will transition to a depolymerization state, rapidly shrinking its length (a process designated as “catastrophe”). MTs show stochastic transitions between periods of shrinkage (catastrophe) and growth (rescue) in a process designated “dynamic instability”. A schematic representation of the tubulin folding and native dimer disassembly pathways is also shown. The CCT (cytosolic chaperonin-containing TCP1) captures tubulin folding intermediates, with important native-like domain structures, either directly from ribosomes or from the hetero-hexameric chaperone prefoldin (for review [
13]). α- and β-tubulin monomers released from CCT follow different pathways: α-tubulin is captured by cofactor B (TBCB) and β-tubulin by cofactor A (TBCA). Then, cofactors E (TBCE) and D (TBCD) capture α- and β-tubulin, respectively. Additionally, after TBCC binding, a supercomplex is formed. TBCC stimulates GTP hydrolysis by β-tubulin and the consequent release of α/β-tubulin-GDP heterodimers. Upon exchange of GDP by GTP, a functional α/β-tubulin dimer competent to polymerize into a MT is formed. Along with tubulin folding, tubulin cofactors assist tubulin heterodimer assembly/dissociation, as well as tubulin degradation. Tubulin heterodimers released from MTs can be dissociated by cofactors and recycled or degraded. TBCD and TBCE are capable of dissociating the tubulin heterodimer by themselves, but in the case of TBCE, its dissociation activity is highly increased by the presence of TBCB. In this process, the β-tubulin is retained by TBCD, whereas α-tubulin is stabilized by the complex TBCB/TBCE. TBCA mainly receives β-tubulin from the dissociation of pre-existing heterodimers instead of newly synthesized tubulins. Both pathways may lead to the tubulin monomer degradation through the ubiquitin-proteasome system by unknown mechanisms. By recycling the tubulin heterodimers, the TBCE/TBCB+TBCA system is crucial for controlling the critical concentration of free tubulin heterodimers and MT dynamics in the cells [
14]. TBCD activity is regulated by Arl2, a small GTP (guanosine triphosphate) binding protein of the Arf family. The figure was inspired on [
15,
16].
3. The Functional Diversity of Microtubules and Microtubule-Based Structures
Microtubules play a myriad of functions in eukaryotes cells and are the components of complex structures, such as the mitotic spindles involved in cell division, centrosome/centrioles that are MT and actin organizing centers, and cilia that can generate motility and have sensory functions coupled with a variety of signaling pathways. The dynamic MT behavior allows MT ends to explore the cell space searching for and binding to intracellular structures (e.g., chromosomes during mitosis) and organelles, such as mitochondria and melanosomes, contributing to their dynamic positioning and organization of the cytoplasm [
4,
17,
18,
19]. Additionally, MTs are targeted toward focal adhesions by interacting with actin and intermediate filaments, and their plus ends are then captured and anchored to the cell cortex near these structures [
20]. The turnover of focal adhesions is dependent on MTs, although the mechanisms have not been completely elucidated. However, it is well established that MTs are involved in the transport of exocytic vesicles containing cargos that are delivered near focal adhesions and control endocytosis and integrin internalization [
21,
22]. Consequently, MT networks regulate cellular adhesion to the extracellular matrix and cell migration. Cell migration is also essential in wound healing/tissue repair, tissue renewal, and during immune responses and angiogenesis, and it contributes to metastasis in several types of cancers [
23,
24,
25,
26].
In crosstalk with actin and intermediate filaments, the MT cytoskeleton also dynamically organizes the cytoplasm space and is involved in cell shape, which can be quickly remodeled in response to internal and external cues [
27]. This is clearly illustrated by different events dependent on MT dynamics occurring during development, such as cell division, cell migration, cell polarization, and differentiation, with implications in cell fate and morphogenesis.
Polarity can be viewed as the asymmetric spatial organization and localization of biomolecules and cellular components (e.g., membrane domains, organelles such as the Golgi apparatus, mitochondria, centrosomes, cilia, and others) that originates structural/functional asymmetries [
28,
29,
30]. The establishment of polarity is important for biological behavior at the individual cell level and for the three-dimensional organization of tissues and organs. For example, epithelial cells are permanently polarized, possessing basolateral surface domains characterized by distinct compositions of proteins and lipids that are established and maintained by tight junctions. During their morphogenesis, MTs undergo a dramatic remodeling changing from an aster organized by the centrosome to a non-centrosomal network that aligns along the apical-basolateral polarity axis [
31,
32]. Recently, it was shown that these MT arrays are able to bear compressive forces, and the cells are shorter in the absence of these MT-based forces. Moreover, the fact that MTs coupled with adherens junctions through the fat planar cell polarity signalling pathway allows the patterns of these forces to travel through the epithelia [
33]. This shows that individual cell MT organization regulates not only cell response to forces, but also coordinates the collective response of cells during tissue morphogenesis [
33].
Another example of MT cytoskeleton remodeling assisting morphogenesis is illustrated by platelets’ formation from their precursor cells, the megakaryocytes. These cells are characterized by membrane structures, called the demarcation membrane system, required for the elongation of proplatelet shafts, as well as protrusions that then shed the proplatelets from their tips (for review [
34]). The formation of proplatelets requires dynamic remodeling and profound changes in the actin and MT cytoskeleton, such as the continuous growth of MT plus-ends and sliding of adjacent MTs [
35]. Platelet size is limited by MT bundling, elastic bending, and actin–myosin–spectrin cortex forces [
36].
Cilia biogenesis is also accompanied by cytoskeleton remodeling. During primary cilia assembly, the centrosome leaves its position at the cell center and migrates toward the cell membrane. During this migration, the older centriole (mother centriole) undergoes a complex conversion process into a basal body that finally anchors to the plasma membrane via distal appendages and assembles the MT ciliary axoneme [
37]. The centrosome migration is driven by the cytoskeleton remodeling characterized by increased MT nucleation and/or stabilization and bundling, accompanied by a contraction and symmetry breaking of the actin network [
38]. Microtubules assemble into a large bundle oriented between the centrosome and the cell’s basal pole, pushing the centrosome toward the apical membrane. The distal appendage protein Cep164 appears to be a critical player in MT cytoskeleton remodeling during centrosome migration [
38].
4. In Vivo Microtubule Dynamics Is Modulated by Microtubule-Associated Proteins
In all of the referred processes, involving MTs, cells show the ability to modulate and explore their dynamic instability and organization by regulating the interaction of these polymers with a large family of motor proteins that produce forces and movement and other MT-associated proteins (MAPs) [
39,
40]. For example, in cell division, an overall dramatic reorganization of the interphase MT array that culminates with the mitotic spindle requires the combined action of MAPs, including motor proteins [
41].
In interphase cells, centrosomes are usually located at the cell center in close association with the nucleus [
42]. This localization is dynamic and, together with the nucleus position, constitutes a primary polarity axis for organelle organization in the cytoplasm. One of the important factors acting in the complex landscape of centrosome positioning is the balance of pushing and pulling forces acting on the centrosome generated by MT dynamics, MAPs, including motors (e.g., kinesins and dyneins), as well as specialized cell cortex anchor sites [
43,
44,
45,
46,
47,
48,
49]. On the other hand, nucleus positioning also depends on pushing forces [
50,
51,
52]. Consequently, the overall MT organization plays a pivotal role in the spatial distribution of pushing forces [
53,
54], and it changes in this organization, which will influence the positions of the centrosome and the nucleus.
MAPs are a vast and complex family of distinct proteins that either bind through and stabilize the MT lattice, e.g., Tau, MAP2, and MAP4, or bind to the MTs ends, e.g., the plus tip-binding proteins (+TIPs), EB1 [
55], and the CAP-GLY-containing proteins, e.g., the +TIP cytoplasmic linker protein CLIP-170. Plus-end tracking proteins (+TIPs) are specific MAPs that are conserved in all eukaryotes and specifically accumulate at the growing MT plus ends and regulate MTs dynamics. MTs interact with cellular structures, membranes, signaling factors, and the forces exerted in MT arrays [
56]. MAPs’ roles have an impact on various critical cellular activities, such as intracellular transport, cell division, polarity establishment, cell motility, and morphogenesis. Among +TIPs, the end-binding protein (EBs) family tracks the growing MTs ends and regulates MT dynamics both directly and by recruiting a variety of other unrelated +TIPs, such as CAP-Gly-containing proteins (CLIP-170, CLIP-115, p150Glued) [
57,
58]. In vitro, EB1 increases MT nucleation and growth rate and promotes both catastrophes and rescues [
55,
59,
60]. EB proteins possibly bind to the tubulin-GTP (or GDP-Pi) MT cap [
61,
62,
63], and the size of the EB binding region tends to decrease [
61] prior to MT transition from growth to depolymerization. Therefore, long binding regions of EB proteins originate protective caps that stabilize the MT [
64]. Other +TIPs, e.g., CLASPs, spectraplakins, and APC, which usually act as MT-stabilizing factors, are involved in MT capture and stabilization near the leading edge of migrating cells [
65,
66,
67]. By contrast, the +TIP kinesin-13 family member can remove tubulin subunits by the tip hydrolyzing ATP and promoting catastrophe [
68]. Other MAPs can also promote MT depolymerization, such as stathmin, which sequesters tubulin heterodimers, increasing MT catastrophe [
69]. Stathmin also binds to protofilaments exposed at the tips of growing MTs and directly promotes catastrophe, at least in part, by interfering with lateral bonding between subunits [
70].
Recently, MAPs that stabilize MT dynamic properties (e.g., XMAP215, TPX2, and CAMSAP/Patronin) have been found to contribute to MT nucleation, a role mainly attributed to the γ-tubulin ring complex (for review [
71]). MAPs also comprise the MT-severing enzymes, namely, katanin [
72], spastin [
73,
74], and fidgetin [
75], which are members of the meiotic subfamily of AAA ATPases [
76,
77]. These enzymes create internal damage in the MT lattice by active extraction of tubulin heterodimers, causing depolymerization and catastrophe [
75,
78], and they play important roles, as, for example, in cell division [
79], neurogenesis [
80,
81,
82], and cilia biology [
83]. More recently, MT-severing enzymes started to emerge as able to amplify MT arrays by promoting the incorporation of GTP-tubulin throughout the MT lattice, as well as by promoting the regrowth of severed MTs, increasing the number and the mass of these polymers [
76,
84]. Similar behavior was observed for the +TIP CLIP-family members that can function as rescue factors for shrinking MTs [
85]. The roles played by MAPs are not limited to the regulation of MTs stability/dynamics and linking with cellular structures, since they are also involved in the control of MT architecture by regulating, for example, MT spacing [
86,
87,
88], cross-talk of MTs with other cytoskeleton filaments (for review [
40]), control of MT protofilament numbers (doublecourtin) [
89], regulation of motor motility [
90], and in the cross-linking of adjacent MTs promoting bundling. For example, the PRC1 protein (MAP65) crosslinks antiparallel MTs [
91] that are required for cytokinesis at the end of anaphase at the spindle midzone [
92]. The PRC1 protein recruits to this region the Xklp1 kinesin (Xenopus kinesin-4) that guarantees the maintenance of the size of the MTs overlap [
92].
More recently, using Cryo-ET, it has been shown that the MT doublets of the motile ciliary axonemes have inner protein structures that periodically bind inside its lumen, creating a sheath (for review [
93]). Although the exact role of these MT inner proteins (MIPs) is not yet completely elucidated, they establish a unique architecture of the MT doublet, stabilizing it against strong mechanical forces during cilia beating [
93,
94]. Additionally, their organization and periodicity limit the MT protofilaments that can be used for the intraflagellar transport (IFT) required for the assembly and maintenance of cilia [
95].
In addition, diverse proteins, which can bind to MTs, are involved in signal transduction, protein translation, and metabolism [
96]. Finally, it is observed that mechanical forces produced by kinesin and dynein MT motor proteins can remove tubulin dimers from the lattice and destroy MTs [
97,
98]. Triclin and coworkers (2021) showed the existence of a MT self-repair mechanism where tubulin heterodimer removal can be compensated for the insertion of free tubulin dimers into the MT lattice [
98]. It has been proposed that MAPs may play a role in this process, maintaining the MT lattice integrity [
40]. The vast functional diversity of MAPs suggests that distinct MT arrays may be regulated by different balances of distinct MAPs and respond to different MAPs networks that can locally originate distinct environments in response to various signals. MT recognition/binding by MAPs may be affected by diverse post-translational modifications (PTMs) of tubulin or by incorporating distinct ratios of different tubulin isotypes [
99].
5. The In Vivo Diversity of Tubulin Pools and Microtubule Functional Diversity
The biochemical diversity of tubulin pools is generated by a combination of different tubulin isotypes encoded by the distinct tubulin gene family members, which may exhibit constitutive, developmental, and tissue-specific expression patterns, as well as by diverse PTMs that tubulin can experience [
15,
100]. There is growing evidence that specific tubulin isotypes encoded by members of tubulin multigenic families may be required to assemble functional distinct MT structures [
101,
102]. For example, the product of the β2-gene of
Drosophila is important for the correct axonemal structure in sperm, and males expressing mutations in this gene are infertile [
103,
104]. Moreover, in
Caenorhabditis elegans, α- and β-tubulin (coded by genes
mec-12 and
mec-7, respectively) are specific for the assembly of MTs, owning to 15 protofilaments that are found in the touch receptor neurons, showing that this specific α-tubulin isotype is required for the assembly of a specific class of neuronal MTs [
105,
106]. In mammals, the divergent β1-tubulin isotype is expressed exclusively in platelets and megakaryocytes. β1-tubulin has a specialized role in platelet synthesis, structure, and function, and it is required to maintain the high degree of MT bundling and elastic bending necessary for the specialized MT arrays of platelets [
36,
107].
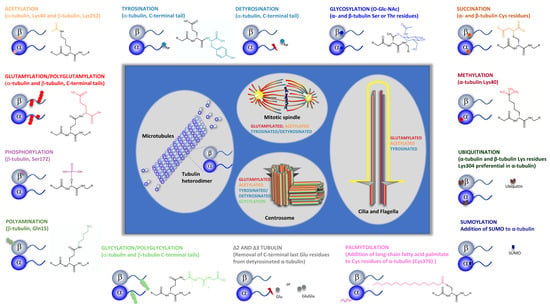
On the other hand, tubulins present a complex pattern of distinct conserved PTMs (See
Figure 2; reviewed in [
108,
109]), namely: (i) phosphorylation, i.e., the addition of a phosphate group to Ser, Thr, or Tyr residues in α- and β-tubulins, including β-tubulin Ser172, β3-tubulin Ser444, α-tubulin Tyr432, and yet not identified α-tubulin and β-tubulin Tyr residues [
110,
111,
112,
113,
114,
115]; (ii) methylation of α-tubulin at Lys40 [
116] and Lys311 and β-tubulin at Lys19 and Lys297 [
117]; (iii) palmitoylation, i.e., addition of long-chain fatty acid palmitate to Lys residues with α-tubulin Lys376 being a major modification site [
118]; (iv) polyamination, i.e., addition of polyamines to the γ- carboxamide group of Gln side chains in α- and β-tubulin, with β-tubulin Gln15 being the major modification site [
119]; (v) tyrosination/detyrosination, i.e., the enzymatic ligation of tyrosine and the enzymatic removal of tyrosine, respectively, which occurs at the α-tubulin C-terminal Tyr residue [
120,
121,
122]; (vi) glycylation and polyglycylation, i.e., the addition of Gly to the γ-carboxy group of Glu side chains and chain elongation by additional addition of Gly residues; multiple Glu residues can be modified in α- and β-tubulin C- terminal tails [
123]; (vii) glutamylation and polyglutamylation, i.e., addition of Glu to the γ-carboxy group of Glu side chains and chain elongation by additional addition of Glu residues; multiple Glu residues can be modified in α- and β-tubulin C-terminal tails [
124,
125,
126,
127]; (viii) ubiquitination, i.e., the addition of ubiquitin to Lys residues of tubulin, with α-tubulin Lys304 being the major modification site [
128,
129]; (ix) sumoylation, i.e., covalent conjugation of SUMO (small ubiquitin-related modifier) [
130]; (x) Creation of Δ2-tubulin and Δ3-tubulin by removal of C-terminal penultimate Glu residues from detyrosinated α-tubulin [
131,
132,
133] and (xi) acetylation, i.e., the addition of an acetyl group to Lys residues, with at least 12 sites of acetylation in α-tubulin identified by proteomic studies [
134,
135,
136,
137,
138,
139] (for details, see the next sections). Tubulin succination, a modification that occurs when fumarate reacts with cysteine residues to generate S-(2-succino)cysteine [
140], and the α- and β-tubulin glycosylation of Ser or Thr residues by the binding of O-linked β-N-acetylglucosamine (O-Glc-NAc)) [
136,
141], are the two less-studied tubulin PTMs (
Figure 2). Although not much is known about succinated tubulin, it seems that this post-translation modification is more abundant in more dynamic MTs [
140]. The in vitro addition of O-Glc-NAcylation to α-tubulin, but not to β-tubulin, causes a decrease in the interactions required for dimer assembly and inhibits tubulin polymerization [
136,
141]. The extensive diversity and complexity of distinct tubulin PTMs led to the proposal that they would create a pattern on the MT surface, which is known as the “tubulin code” [
142].
Figure 2. Tubulin post-translational modifications. Specific groups of functionally distinct MT structures and their more common PTMs are highlighted (i.e., centrosome, cilia, and mitotic spindle). Specific tubulins amino acid residues or tubulin specific domains where PTMs occur are indicated. PTMs chemical structure are specified. Tubulin PTMs caused by association with specific proteins, such as SUMO and ubiquitin, as well as those originated by specific proteolysis (detyrosination and Δ2 and Δ3 tubulin) are also shown. For Glutamylation PTM, only the chemical structure corresponding to monoglutamylation is shown.
Tubulin PTMs are reversible and regulated and, in the last years, many of the enzymes that catalyze the formation or removal of these modifications have been identified [
3,
108,
143]. Interestingly, the enzymes that catalyze the formation of PTMs seem to have a catalytic preference for MTs over free tubulin as substrates. This preference for MTs is illustrated by the carboxypeptidase catalyzing tubulin detyrosination [
144], the specific acetyltransferase α-tubulin acetyltransferase 1 (αTAT1), which catalyzes α-tubulin Lys40 acetylation, as well as tubulin polyglutamylase [
145,
146]. On the contrary, the enzymes that remove tubulin PTMs, such as deglutamylases, can catalyze deglutamylation on both polymerized and soluble tubulin, whereas human histone deacetylase 6 (HDAC6), the major deacetylase catalyzing the removal of the acetyl group from α-tubulin Lys40, prefers tubulin dimers as substrates [
147,
148], and tubulin-tyrosine ligase (TTL) exclusively uses tubulin dimers as substrates [
149,
150]. One exception to these observations is the phosphorylation of β-tubulin Ser172, catalyzed by the cyclin-dependent kinase Cdk1 during the transition from interphase to mitosis and by the MNB/DYRK1a kinase, which regulates MT dynamics in neurons and which occurs mainly at the polymer and inhibits the polymerization of the heterodimers [
110,
111].
Although it is known that tubulin can undergo a large variety of rapid and reversible PTMs, some of which have been studied in depth, the knowledge about the crosstalk between those modifications is still sketchy. A possible crosstalk between tubulin glycylation and glutamylation has been suggested [
151]. These PTMs occur within the same cluster of glutamate residues, which may indicate a possible competition between tubulin glycylation and glutamylation. In
Tetrahymena and
Drosophila, mouse loss of glycylation is accompanied by tubulin hyperglutamylation, indicating that both PTMs are possibly regulated together [
152,
153,
154]. Another crosstalk is that between methylation and acetylation, since there is an obvious competition for the same α-tubulin lysine residue (Lys40) in MTs, which can undergo either acetylation or methylation catalyzed by the methyltransferase SET domain containing 2 (SETD2) [
116]. The stoichiometry of Lys40 methylation and acetylation within MTs is not known, but a recent study in mouse cortical neurons showed, as expected, an inverse relationship between the levels of MT Lys40 trimethylation (which decreases between embryonic day 17.5 and adulthood) and Lys40 acetylation (which increases during this period) [
155]. Moreover, another study showed that α-tubulin Lys40 trimethylation is able to rescue the defects of radial migration and morphological transition of cortical neurons caused by α-tubulin Lys40 acetylation deficiency [
156]. Another crosstalk may exist between tubulin tyrosination and α-tubulin Lys40 acetylation. In fact, a correlation between tubulin acetylation and detyrosination was found in α-TAT1 that is acutely depleted and knock-out murine embryonic fibroblasts, in which both acetylated and detyrosinated MTs levels appeared to be decreased [
157]. More recently, it was found in primary neurons that tubulin re-tyrosination can control acetylated tubulin levels and that tubulin acetylation is affected by the tubulin tyrosination/detyrosination cycle [
158]. Interestingly, a crosstalk between the tubulin tyrosination/detyrosination cycle and neuron-specific Δ2 tubulin also occurs. The pool of detyrosinated tubulin can be further acted upon by cytosolic carboxy peptidases (CCPs), which catalyze the removal of the terminal glutamate residue from tubulin, converting detyrosinated tubulin into Δ2 tubulin [
159]. Given that the enzyme catalyzing tubulin tyrosination, tubulin tyrosine ligase, is unable to use Δ2 tubulin as substrate, the formation of Δ2 tubulin is irreversible and, besides removing it from the tubulin tyrosination/detyrosination cycle [
150], may also affect the levels of α-tubulin acetylation.
Since formation/removal of tubulin modifications can occur in free tubulin heterodimers or MTs, we may wonder if tubulin co-factors play a regulatory role in remodeling tubulin PTMs patterns in MTs in response to specific cues by regulating tubulin recycling, degradation, and quality control. Undoubtedly, the set of enzymes that catalyze and revert tubulin PTMs and their patterns and mechanisms of regulation are another complex layer in the regulation of MT organization, function, and dynamics, with profound importance for cell homeostasis. Thus, it is expected that abnormal tubulin posttranslational modifications may contribute to various human diseases, such as cilia, neuronal, muscle, blood disorders, and cancer (reviewed here and in [
160]).
The combination of tubulin isotypes and isoforms patterns contributes to modulating/changing/fine-tuning intrinsic features of MTs, such as stability and dynamics, mechanical properties (flexibility and resistance to mechanical stress), polymerization/depolymerization rates, and the ability to form specific MTs organizations and structures. Additionally, these patterns probably affect the interactions of MTs with motor proteins and MAPs, which will also have consequences on MTs’ intrinsic properties. The generated diversity will allow cells to cope with different environments, signals and, in the case of metazoans, with different tissue architectures and physiology challenges. Supporting these ideas is the fact that distinct patterns of tubulin isotype expression and tubulin PTMs can be observed in different cell types during the cell cycle and development [
161]. Tubulin PTMs may occur and be predominant in specific sets of MTs that coexist inside the same cell. Therefore, specific tubulin PTMs profiles can be found in MTs of centrioles, cilia/flagella, kinetochore fibers, midbody, and axonal and cone MTs (for review [
161]), or even between adjacent MTs, as in the case of the A and B tubules of axonemal doublets. In flagella/cilia of
Chlamydomonas and
Tetrahymena, the B tubule is highly glutamylated [
162,
163], whereas, in algae A, the tubule is enriched by detyrosinated tubulin [
164].