Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Neurosciences
Holoprosencephaly (HPE) is the most common malformation of the prosencephalon in humans. It is characterized by a continuum of structural brain anomalies resulting from the failure of midline cleavage of the prosencephalon. The three classic subtypes of HPE are alobar, semilobar and lobar.
- holoprosencephaly
- nervous system malformation
- craniofacial abnormalities
1. Introduction
Holoprosencephaly (HPE) is characterized by a continuum of structural anomalies of the brain resulting from the failure of differentiation and midline cleavage of the prosencephalon (i.e., forebrain) during the third to fourth weeks of gestation [1,2,3,4,5,6]. HPE is the most common malformation of the prosencephalon in humans. It affects 1:250 conceptuses; however, given the significant number of fetal deaths, its prevalence in live births is lower, ranging from 1:8000 to 1:16,000. This rate appears to be consistent across different populations [7,8].
The degrees of severity of HPE are defined by the extent of the brain malformation, and are classically divided into alobar, semi-lobar, and lobar forms. A spectrum of midline craniofacial defects are associated with HPE [2]. The severity of the clinical phenotype usually mirrors the radiologic and associated facial features [2]. Important advances in the understanding of the etiologic factors, especially the genetic basis, have been achieved in recent decades [9].
2. Overview of Prosencephalic Development
Prosencephalic development consists of three sequential phases that usually overlap, namely, the formation, cleavage, and midline development [10]. Soon after the closure of the anterior neuropore, between days 22 and 24 of gestation, the embryonic prosencephalic vesicle is established at the rostral end of the neural tube. As the prosencephalon undergoes expansion, the cells located at the midline undergo high levels of death and reduced proliferation leading to the splitting of the expanding telencephalon (which will give rise to the cerebral hemispheres) and the diencephalon (which will give rise to the thalamus) [1].
Normal face and brain development require an adequate balance of dorsalizing and ventralizing factors in the developing prosencephalon. Key signaling molecules for the patterning of the prosencephalic midline include bone morphogenic proteins (BMPs), wingless-integrated proteins (WNTs), fibroblast growth factors (FGF) and sonic hedgehog proteins (SHH), which are secreted in the dorsal, rostral and ventral midlines areas, respectively. SHH is a secreted protein that has a key role in the maintenance of the notochord and the patterning and induction of the ventral forebrain [11]. Hedgehog (Hh) signal transduction requires the integrity of the cilia, microtubule-based organelles that project from the cell’s surface, helping the traffic of the Hh pathway proteins [12]. Disruption of midline patterning, as seen in HPE, results in the failure of prosencephalon cleavage into distinct right and left hemispheres. Deep brain structures, and the olfactory and optic bulbs and tracts, can also be affected [3,4,5,6].
The development of the face occurs simultaneously with the formation of the forebrain and relies on signaling from the ventral midline mediated by SHH. The cranial neural crest cells, which are derived from the ectoderm situated at the dorsal border between the neural tube and surface ectoderm of the embryo, migrate towards the pharyngeal arches and the frontonasal process to form the tissues of the skull, the upper cervical tract, and the face [13]. Disruption of this process results in craniofacial defects, as seen in patients with HPE [2]. In cases of severe holoprosencephalies, the premaxillary segments of the face remain unformed, causing midline facial defects, such as arrhinia, midline facial clefts, hypotelorism, and cyclopia [11,14].
3. Radiologic Classification of HPE
The widely accepted classification system for HPE was proposed by DeMyer, who divided holoprosencephaly into three subcategories: alobar, when there is a complete lack of separation of the cerebral hemispheres and a large monoventricle (Figure 1a–d); semi-lobar, with only the anterior lobes failing to separate but the parieto-occipital regions divided by the interhemispheric fissure and falx cerebri (Figure 1e–g); and lobar, when only the most rostral-inferior parts of the frontal lobes are fused [15,16].
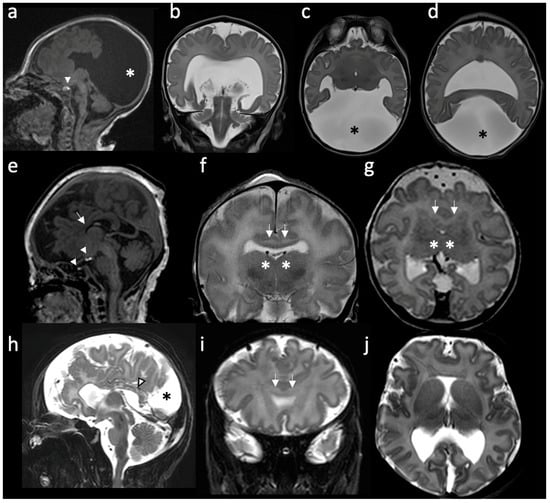
Figure 1. Radiologic features of holoprosencephalies: (a–d) Radiologic features of alobar holoprosencephaly, 2 days old. (a) Sagittal T1-weighted image shows hypodevelopment of the parietal lobes with absence of identifiable occipital lobes and corpus callosum. The bright T1 spot of the neurohypophysis is preserved and well placed (white arrowhead). (b) Coronal T2-weighted image shows the frontal lobes fused across the midline and a large supratentorial monoventricle. (c,d) Axial T2-weighted images show fused thalami and the monoventricle communicating with a large dorsal cyst (asterisk), noting the absence of the septum pellucidum and rudimentary formation of the temporal horns. (e–g) Radiologic features of semilobar holoprosencephaly, 3 days old. (e) Sagittal T1-weighted image shows hypoplastic frontal lobes, absence of anterior corpus callosum (white arrow) and abnormal finding of partial ectopic neural hypophysis associated with residual hyperintense signal seen within the sella (white arrowheads). (f) Coronal T2-weighted image shows fusion of the frontal lobes (white arrows) and partial fusion of thalami (asterisk). (g) Axial T2-weighted image shows that the division of the ventricles is only seen posteriorly. Septum pellucidum is absent. (h–j) Radiologic features of middle interhemispheric variant holoprosencephaly, 15 days old. (h) Sagittal T2-weighted image shows absent body of the corpus callosum but with the presence of the splenium (white arrowhead). Dorsal interhemispheric cyst is present (black asterisk). (i) Coronal T2-weighted image shows fusion of the frontal lobes across the midline (white arrows). Note that the degree of fusion is less extensive than the one seen in the semilobar HPE. (j) Axial T2-weighted image shows fused frontal lobes, absent septum pellucidi and the interhemispheric cyst in the occipital region.
Since then, a few more categories have been added to the original classification. The middle interhemispheric (MIH) variant is characterized by the separation of the anterior and occipital regions of the brain’s hemispheres, while the posterior frontal and parietal lobes remain fused [17] (Figure 1h–j). Milder, minimal and microforms of HPE are also described and are, respectively, associated with septo-optic dysplasia, nonseparation of the preoptic area, and only facial features (hypotelorism and a single maxillary central incisor) with normal brain development [10,15,18]. The radiologic features of holoprosencephalies are summarized in Table 1.
Table 1. Radiologic classification of holoprosencephaly.
| Type | Main Features | References |
|---|---|---|
| Alobar | Absent separation of the cerebral hemispheres; Single “monoventricle”; Agenesis of the corpus callosum, absent third ventricle; Fusion of thalami and basal ganglia; Dorsal cyst is frequent; Significant midline facial defects. |
[6,14,16,19,20] |
| Semilobar | Anterior lobes fail to separate; Interhemispheric fissure detected only posteriorly; Small, partially-formed third ventricle is often noted; Dorsal cyst may also be present; Midline craniofacial defects may be present or only subtle facial abnormalities. |
[6,16] |
| Lobar | Only the most rostral-inferior parts of the frontal lobes are fused; Septum pellucidum is usually absent; Posterior half of the corpus callosum is formed; Varying degrees of basal ganglia and thalamic fusion; Midline craniofacial defects often absent or mild. |
[6,16,21] |
| Middle interhemispheric variant (syntelencephaly) | Failure of separation of the posterior frontal and parietal lobes; Variable lack of cleavage of the basal ganglia and thalami; Absence of the body but presence of the genu and splenium of the corpus callosum. |
[6,16,17,22] |
| Septopreoptic (minimal form) |
Midline fusion restricted to the septal region or preoptic region of the telencephalon. | [16] |
| Microform | Only HPE-related subtle craniofacial anomalies; No structural brain defects on imaging. |
[16] |
It is fundamental to keep in mind that holoprosencephalies represent a continuum of forebrain malformations and no clear distinctions among these different categories should be expected [23,24]. In some cases of lobar HPE, the fusion between the frontal lobes may be minimal, making it particularly difficult to differentiate between a mild lobar HPE and other midline malformations (such as septo-optic dysplasia or isolated fusion of the fornices).
This entry is adapted from the peer-reviewed paper 10.3390/children10040647
This entry is offline, you can click here to edit this entry!
