Traditionally, persimmon leaves (PL) are used as a functional tea in Asian culture to cure different ailments, and are also incorporated into various food and cosmeceutical products as a functional ingredient. PL mainly contain flavonoids, terpenoids, and polysaccharides, along with other constituents such as carotenoids, organic acids, chlorophylls, vitamin C, and minerals. The major phenolic compounds in PL are proanthocyanidins, quercetin, isoquercetin, catechin, flavonol glucosides, and kaempferol. Meanwhile, ursolic acid, rotungenic acid, barbinervic acid, and uvaol are the principal terpenoids. These compounds demonstrate a wide range of pharmacological activities, including antioxidant, anticancer, antihypertensive, antidiabetic, anti-obesity, anti-tyrosinase, antiallergic, and antiglaucoma properties. PL contain a high amount of flavonoids (e.g., astragalin, hyperin, isoquercitrin, kaempferol, and quercetin) and terpenoids along with other compounds, including chlorophylls, carotenes, kryptoxanthin, cellulose, hemicelluloses, and lignins. These compounds exhibit potential antioxidant, antihypertensive, anti-inflammatory, anticancer, antidiabetic, antiallergic, and antimicrobial effects.
- persimmon leaf
- Diospyros kaki L.
- flavonoids
- terpenoids
1. Phenolic Compounds
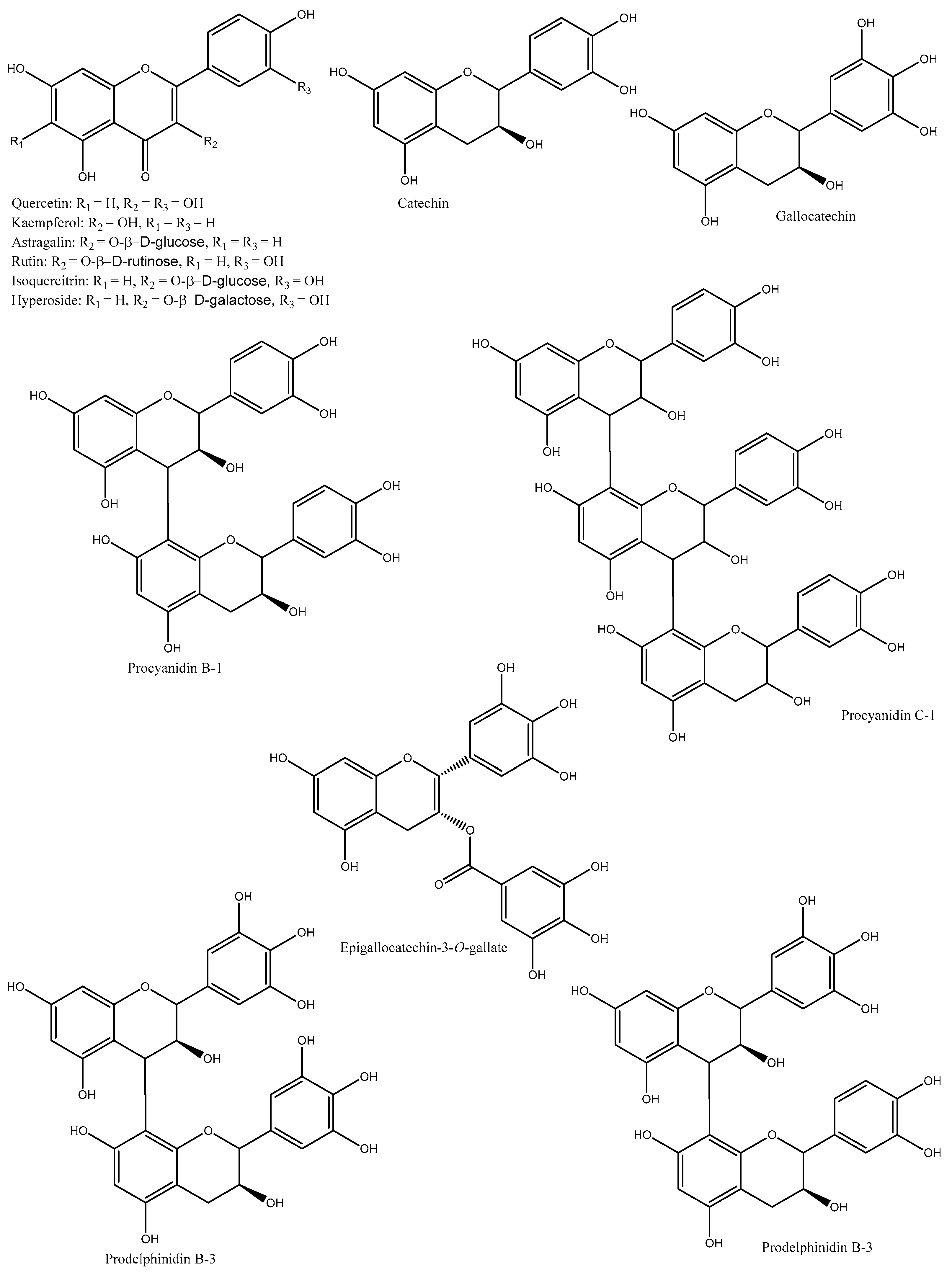
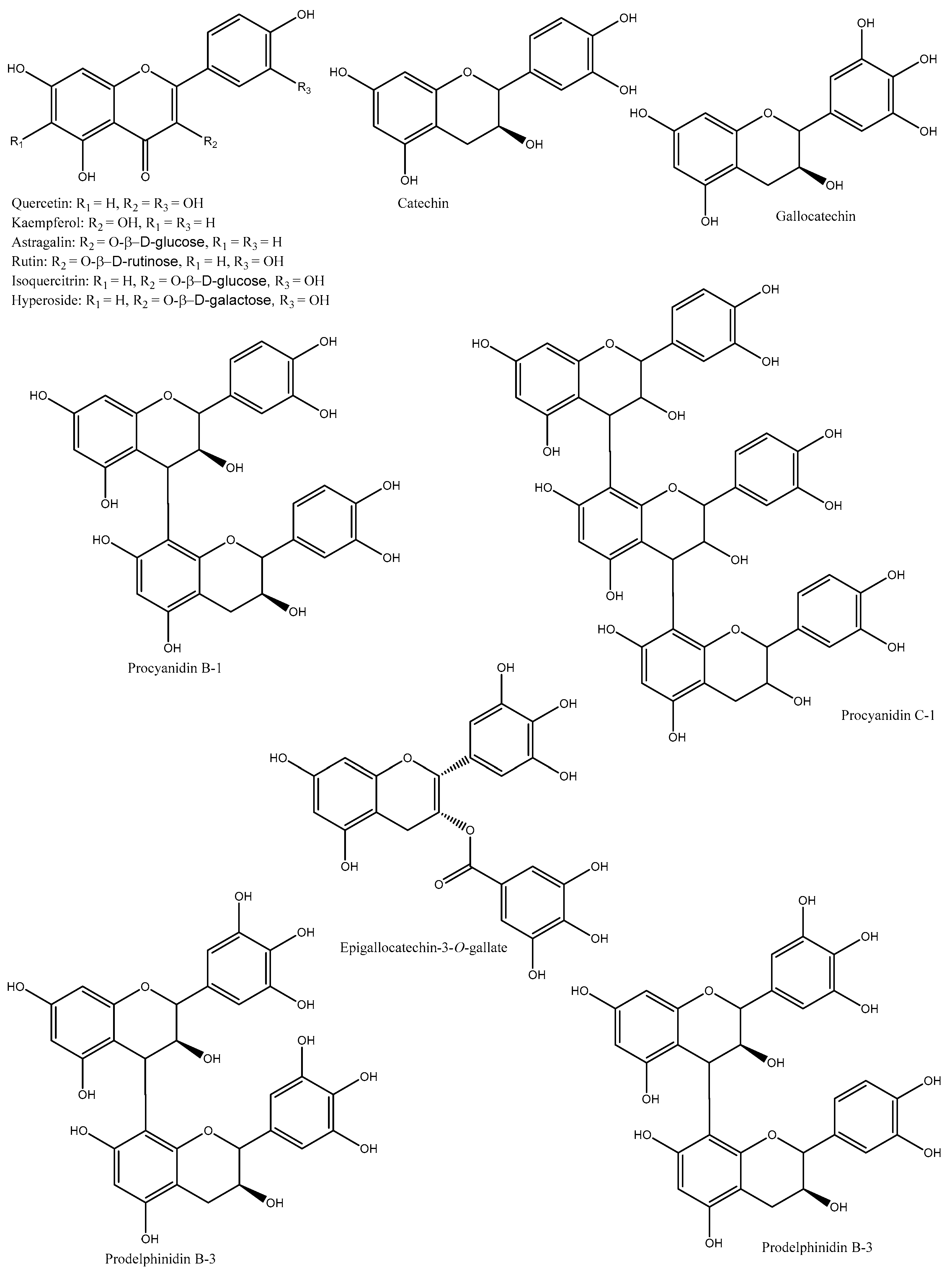
| Species/Cultivars | Origin | TPC (mg GAE/g) | TFC (mg CE/g) | TTC (mg CE/g) | Individual Compounds (µg/g) | References |
|---|---|---|---|---|---|---|
| D. kaki (Sangju dungsi) | Korea | 90.41 | 30.67 | 47 | NA | [6] |
| D. kaki (Sangju-dungsi, Sangamdungsi, Gabjubaekmok, Cheongdobansi, and Suhong) | Korea | 72.59–112.09 | 30.27–37.83 | 28.67–81.33 | NA | [7] |
| D. lotus (Dongsi) | Korea | 58.01–58.16 | 14.16–15.83 | 32.38–35.46 | NA | [8] |
| D. kaki | Korea | NA | NA | NA | Catechin, gallocatechin, pyrocyanidin C-1, procyanidin B-1, prodelphinidin B-3, procyanidin B-7-3-O-gallate, gallocatechin-(4α→8)-catechin, procyanidin C-1-3′-3″-3″-O-trigallate, and epigallocatechin-(4β→8)-epigallocatechin-(4β→8)-catechin | [9] |
| D. kaki | Korea | NA | NA | NA | Quercetin, quercetin-3-O-β-glucoside, quercetin-3-O-β-galactoside, quercetin-3-O-β-2″-galloylglucoside, kaempferol, kaempferol-3-O-β-glucoside, kaempferol-3-O-β-galactoside, and kaempferol-3-O-β-2″-galloylglucoside |
[10] |
| D. kaki | Korea | NA | NA | NA | Isoquercetin, quercetin 3-O-β-D-glucopyranoside-2″-gallate, kaempferol 3-O-β-D-glucopyranoside-2″-gallate, and astragalin | [11] |
| D. kaki | Korea | NA | NA | NA | Catechin, hyperoside, quercetin, isoquercitrin, trifolin, astragalin, quercetin-3-O-β-2″-galloylgalactoside, quercetin-3-O-β-2″-galloylglucoside, kaempferol, kaempferol-3-O-β-D-2″-coumaroylgalactoside, kaempferol-3-O-β-2″-galloylgalactoside, kaempferol-3-O-α-arabinoside, and scopoletin | [12] |
| D. kaki (Hiratanenashi and Tonewase) | Japan | 26–27.7 | NA | NA | Proanthocyanidins (catechin, epigallocatechin, epigallocatechin-3-O-gallate, epicatechin, epicatechin-3-O-gallate, and prodelphinidin) |
[13] |
| D. kaki | Japan | 112 | 58.4 | NA | NA | [14] |
| D. kaki (Fuyu, Jiro, Kinsyu, Tanrei, Yotsumizo, and Saijo) | Japan | NA | NA | NA | Isoquercitrin, hyperoside, trifolin, chrysontemin, astragalin, kaempferol-3-O-(2″-O-galloyl-β-D-glucopyranoside), and quercetin-3-O-(2″-O-galloyl-β-D-glucopyranoside) |
[15] |
| D. kaki | China | NA | NA | NA | Quercetin-3-O-β-glucoside, quercetin-3-O-β-galactoside, quercetin-3-(2-galloylglucoside), kaempferol-3-O-β-glucoside, kaempferol-3-O-β-galactoside, and kaempferol-3-(2-galloylglucoside) |
[16] |
| D. kaki (Tonewase, Fuyu, Aoso, Hachiya, Diamond Bull Heart, and Bull Heart) |
Taiwan | 69.27–149.59 | 40.78–90.62 | 12.58–19.23 | Protocatechuic acid, gallic acid, p-hydroxybenzoic acid, vanillic acid, chlorogenic acid, caffeic acid, p-coumaric acid, sinapic acid, catechin, epicatechin, myricetin-3-O-glucoside, myricetin-3-O-rhamnoside, rutin, quercetin-3-O-glucoside, quercetin-3-O-galactoside, quercitrin, quercetin-3-O-arabinoside, kaempferol-3-O-rutinoside, kaempferol-3-O-glucoside, myricetin, naringin, kaempferol-3-O-rhamnoside, isorhamnetin-3-O-rutinoside, naringenin-7-O-glucoside, isorhamnetin-3-O-glucoside, quercetin, kamempferol, apigenin, and isorhamnetin. | [17] |
| D. kaki (Rojo brillante) | Spain | 86 | 22.9 | NA | [18] | |
| D. kaki (Rojo brillante) | Spain | Gallic acid-O-hexoside (10.3), gallic acid (32.5), gallocatechin (442.2), catechin-O-hexoside I (19), procyanidin B1 (203.71), procyanidin dimer I (54.6), catechin (435.2), procyanidin dimer II (22.6), prodelphinidin dimer B3 (24.4), myricetin-O-hexoside I (304.8), myricetin-O-hexoside II (563.4), isoquercetin (247.4), quercetin-O-hexoside (348.8), quercetin-O-pentoside I (31.9), quercetin-O-pentoside II (52), kaempferol-3-O-glucoside (165.3), kaempferol-O-hexoside I (176.8), myricetin (44.7), quercetin (354.7), kaempferol (206.2), and isorhamnetin (42.8) | [19] | |||
| D. kaki | China | Astragalin, trifolin, annulatin, myricetin, myricetin-3-O-glucopyranoside, quercetin, vitexin, hyperoside, quercetin-3-O-galloylglucoside, isorhamnetin-3-β-D-glucopyranoside, isorhamnetin-3-β-D-galactoside, kaempferol, kaempferol-3-O-galloylglucoside, kaempferol-3-O-galloylgalactoside, and salvianolicacid D | [20] |
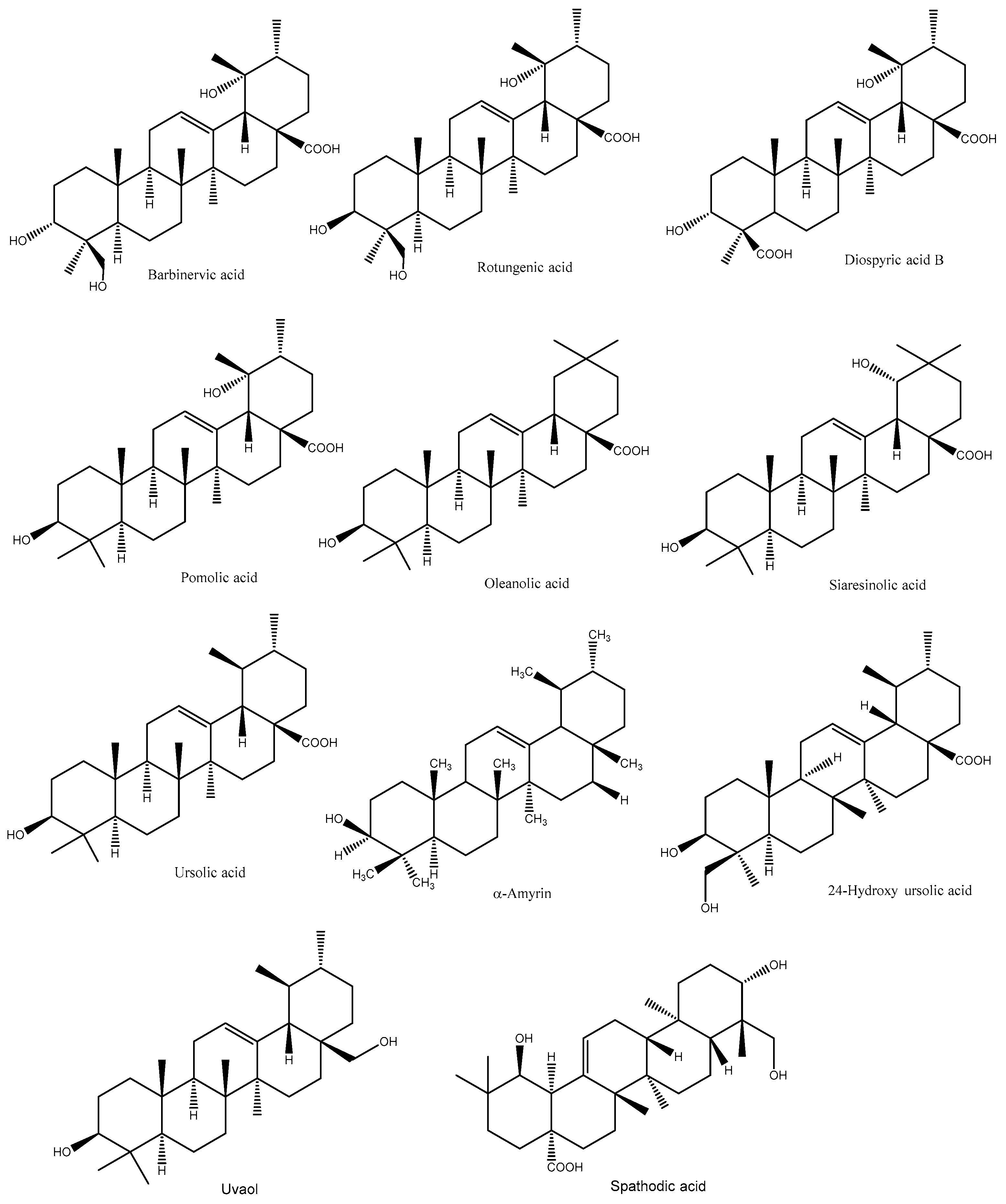
2. Terpenoids
3. Polysaccharides
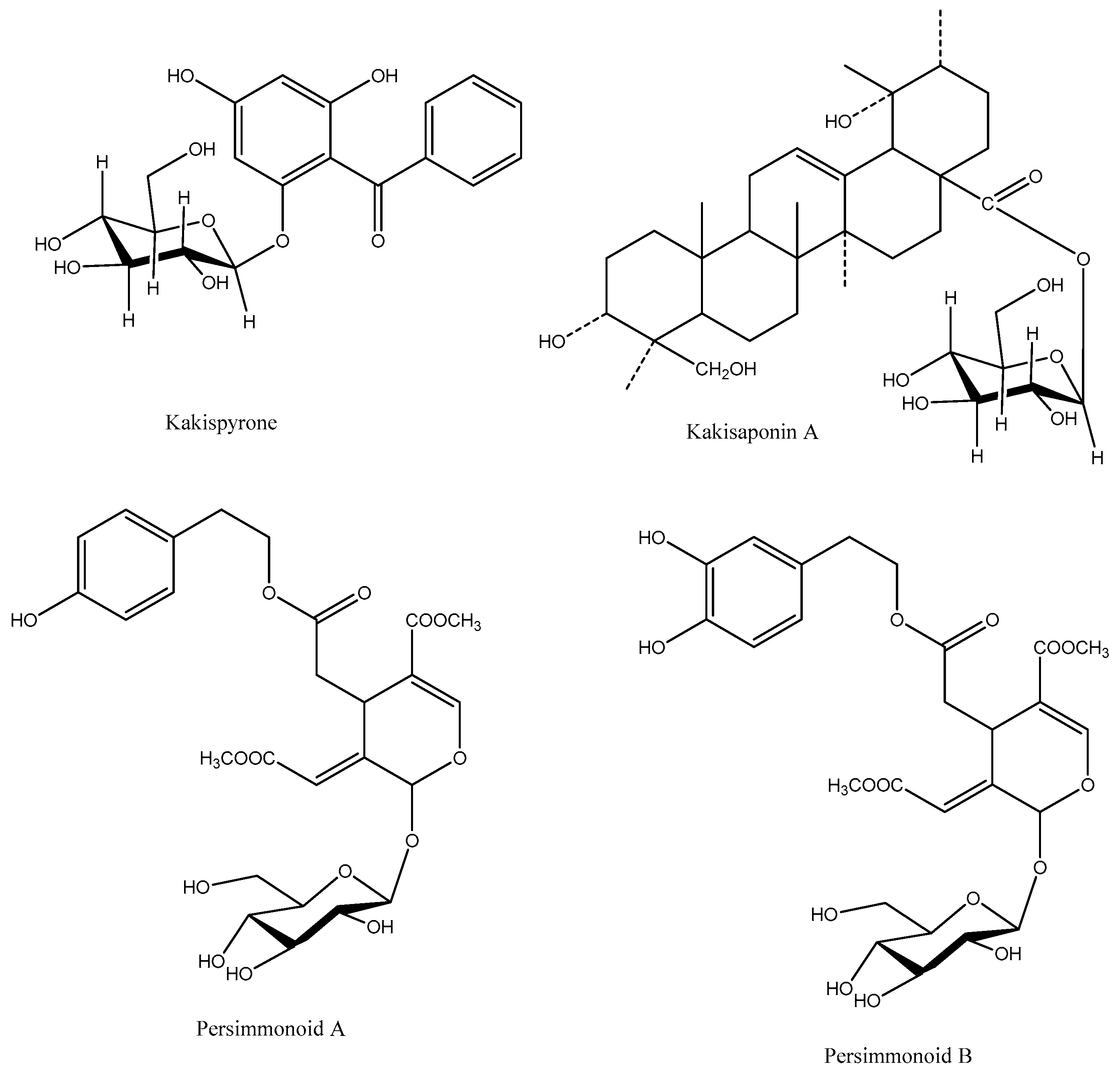
4. Other Compounds
This entry is adapted from the peer-reviewed paper 10.3390/plants12040937
References
- Hossain, A.; Dave, D.; Shahidi, F. Antioxidant potential of sea cucumbers and their beneficial effects on human health. Mar. Drugs 2022, 20, 521.
- Hossain, A.; Dave, D.; Shahidi, F. Effect of High-Pressure Processing (HPP) on Phenolics of North Atlantic Sea Cucumber (Cucumaria frondosa). J. Agric. Food Chem. 2022, 70, 3489–3501.
- Hossain, A.; Yeo, J.D.; Dave, D.; Shahidi, F. Phenolic compounds and antioxidant capacity of sea cucumber (Cucumaria frondosa) processing discards as affected by high-pressure processing (HPP). Antioxidants 2022, 11, 337.
- Hossain, A.; Senadheera, T.R.L.; Dave, D.; Shahidi, F. Phenolic profiles of Atlantic sea cucumber (Cucumaria frondosa) tentacles and their biological properties. Food Res. Int. 2023, 163, 112262.
- Xie, C.; Xie, Z.; Xu, X.; Yang, D. Persimmon (Diospyros kaki L.) leaves: A review on traditional uses, phytochemistry and pharmacological properties. J. Ethnopharmacol. 2015, 163, 229–240.
- Hossain, A.; Moon, H.K.; Kim, J.-K. Effect of pre-treatment and extraction conditions on the antioxidant properties of persimmon (Diospyros kaki) leaves. Biosci. Biotechnol. Biochem. 2017, 81, 2079–2085.
- Hossain, A.; Moon, H.K.; Kim, J.-K. Antioxidant properties of Korean major persimmon (Diospyros kaki) leaves. Food Sci. Biotechnol. 2018, 27, 177–184.
- Hassan, O.; Chang, T.; Hossain, A. Changes in the secondary compounds of persimmon leaves as a defense against circular leaf spot caused by Plurivorosphaerella nawae. PLoS ONE 2020, 15, e0230286.
- An, B.-J.; Choi, H.-J.; Son, J.-H.; Woo, H.-S.; Han, H.-S.; Park, J.-H.; Son, G.-M.; Son, G.-M.; Choi, C. Identification of biologically effect and chemical structure of polyphenol compounds from the leaves of Korea persimmon (Diospyrus kaki L. Folium). J. Korean Soc. Food Cult. 2003, 18, 443–456.
- Kim, K.A.; Kang, S.W.; Ahn, H.R.; Song, Y.; Yang, S.J.; Jung, S.H. Leaves of persimmon (Diospyros kaki Thunb.) ameliorate N-methyl-N-nitrosourea (MNU)-induced retinal degeneration in mice. J. Agric. Food Chem. 2015, 63, 7750–7759.
- Choi, S.; Kang, W.; Chung, S.; Cheon, S. Antioxidative activity of flavonoids in persimmon leaves. Foods Biotechnol. 1996, 5, 119–123.
- Kwon, J.; Park, J.E.; Lee, J.S.; Lee, J.H.; Hwang, H.; Jung, S.H.; Kwon, H.C.; Jang, D.S. Chemical constituents of the leaves of Diospyros kaki (Persimmon). Plants 2021, 10, 3032.
- Kawakami, K.; Aketa, S.; Nakanami, M.; Iizuka, S.; Hirayama, M. Major water-soluble polyphenols, proanthocyanidins, in leaves of persimmon (Diospyros kaki) and their α-amylase inhibitory activity. Biosci. Biotechnol. Biochem. 2010, 74, 1380–1385.
- Sakanaka, S.; Tachibana, Y.; Okada, Y. Preparation and antioxidant properties of extracts of Japanese persimmon leaf tea (kakinoha-cha). Food Chem. 2005, 89, 569–575.
- Xue, Y.L.; Miyakawa, T.; Hayashi, Y.; Okamoto, K.; Hu, F.; Mitani, N.; Furihata, K.; Sawano, Y.; Tanokura, M. Isolation and tyrosinase inhibitory effects of polyphenols from the leaves of persimmon, Diospyros kaki. J. Agric. Food Chem. 2011, 59, 6011–6017.
- Peng, L.; Zhao, M.; Li, H. Method development and validation for simultaneous determination of six flavonoids in rat eyes after oral administration of Diospyros kaki leaves extract by UPLC-MS/MS. Chem. Pharm. Bull. 2021, 69, 218–221.
- Chang, Y.L.; Lin, J.T.; Lin, H.L.; Liao, P.L.; Wu, P.J.; Yang, D.J. Phenolic compositions and antioxidant properties of leaves of eight persimmon varieties harvested in different periods. Food Chem. 2019, 289, 74–83.
- Martínez-Las Heras, R.; Pinazo, A.; Heredia, A.; Andrés, A. Evaluation studies of persimmon plant (Diospyros kaki) for physiological benefits and bioaccessibility of antioxidants by in vitro simulated gastrointestinal digestion. Food Chem. 2017, 214, 478–485.
- Las Heras, R.M.; Quifer-Rada, P.; Andrés, A.; Lamuela-Raventós, R. Polyphenolic profile of persimmon leaves by high resolution mass spectrometry (LC-ESI-LTQ-Orbitrap-MS). J. Funct. Foods 2016, 23, 370–377.
- Huang, S.W.; Wang, W.; Zhang, M.Y.; Liu, Q.B.; Luo, S.Y.; Peng, Y.; Sun, B.; Wu, D.L.; Song, S.J. The effect of ethyl acetate extract from persimmon leaves on Alzheimer’s disease and its underlying mechanism. Phytomedicine 2016, 23, 694–704.
- Tao, W.; Pan, H.; Jiang, H.; Wang, M.; Ye, X.; Chen, S. Extraction and identification of proanthocyanidins from the leaves of persimmon and loquat. Food Chem. 2022, 372, 130780.
- Cho, Y.J.; An, B.J.; Kim, J.H. Application of isolated tyrosinase inhibitory compounds from persimmon leaves. J. Life Sci. 2011, 21, 976–984.
- Bei, W.; Zang, L.; Guo, J.; Peng, W.; Xu, A.; Good, D.A.; Hu, Y.; Wu, W.; Hu, D.; Zhu, X.; et al. Neuroprotective effects of a standardized flavonoid extract from Diospyros kaki leaves. J. Ethnopharmacol. 2009, 126, 134–142.
- Bae, U.J.; Park, S.H.; Jung, S.Y.; Park, B.H.; Chae, S.W. Hypoglycemic effects of aqueous persimmon leaf extract in a murine model of diabetes. Mol. Med. Rep. 2015, 12, 2547–2554.
- Martínez-Las Heras, R.; Heredia, A.; Castelló, M.L.; Andrés, A. Influence of drying method and extraction variables on the antioxidant properties of persimmon leaves. Food Biosci. 2014, 6, 1–8.
- Kazzem, M.; Sun, Y.T.; Low, M.; Seto, S.W.; Chang, D.; Lee, S.; Suresh, H.; Khoo, C.S.; Bensoussan, A.; Kiat, H. Chromatographic analysis and anti-oxidative property of Naoxinqing tablet, a proprietary preparation of Diospyros kaki leaves. Molecules 2019, 24, 1101.
- Wang, L.; Xu, M.L.; Rasmussen, S.K.; Wang, M.H. Vomifoliol 9-O-α-arabinofuranosyl (1→6)-β-D-glucopyranoside from the leaves of Diospyros kaki stimulates the glucose uptake in HepG2 and 3T3-L1 cells. Carbohydr. Res. 2011, 346, 1212–1216.
- Shahidi, F.; Hossein, A. Importance of insoluble-bound phenolics to the antioxidant potential is dictated by source material. Antioxidants 2023, 12, 203.
- Shahidi, F.; Hossain, A. Bioactives in spices, and spice oleoresins: Phytochemicals and their beneficial effects in food preservation and health promotion. J. Food Bioact. 2018, 3, 8–75.
- Fan, J.P.; He, C.H. Simultaneous quantification of three major bioactive triterpene acids in the leaves of Diospyros kaki by high-performance liquid chromatography method. J. Pharm. Biomed. Anal. 2006, 41, 950–956.
- Chen, G.; Lu, H.; Wang, C.; Yamashita, K.; Manabe, M.; Meng, Z.; Xu, S.; Kodama, H. Effect of five flavonoid compounds isolated from leaves of Diospyros kaki on stimulus-induced superoxide generation and tyrosyl phosphorylation of proteins in human neutrophils. Clin. Chim. Acta 2002, 326, 169–175.
- Chen, G.; Wang, Z.Q.; Jia, J.M. Three minor novel triterpenoids from the leaves of Diospyros kaki. Chem. Pharm. Bull. 2009, 57, 532–535.
- Chen, G.; Ren, H.; Yu, C. A new 18,19-secoursane triterpene from the leaves of Diospyros kaki. Chem. Nat. Compd. 2012, 47, 805–806.
- Phuong, T.T.; Chul, H.L.; Trong, T.D.; Phi, H.N.; Wan, G.K.; Sang, J.L.; Won, K.O. Triterpenoids from the leaves of Diospyros kaki (Persimmon) and their inhibitory effects on protein tyrosine phosphatase 1B. J. Nat. Prod. 2008, 71, 1775–1778.
- Park, J.Y.; Shin, M.S. Inhibitory effects of pectic polysaccharide isolated from Diospyros kaki leaves on tumor cell angiogenesis via VEGF and MMP-9 regulation. Polymers 2021, 13, 64.
- Park, H.R.; Hwang, D.; Do Hong, H.; Shin, K.S. Antitumor and antimetastatic activities of pectic polysaccharides isolated from persimmon leaves mediated by enhanced natural killer cell activity. J. Funct. Foods 2017, 37, 460–466.
- Song, Y.R.; Han, A.R.; Lim, T.G.; Kang, J.H.; Hong, H. Do Discrimination of structural and immunological features of polysaccharides from persimmon leaves at different maturity stages. Molecules 2019, 24, 356.
- Hwang, Y.H.; Ha, H.; Kim, R.; Cho, C.W.; Song, Y.R.; Do Hong, H.; Kim, T. Anti-osteoporotic effects of polysaccharides isolated from persimmon leaves via osteoclastogenesis inhibition. Nutrients 2018, 10, 901.
- Lee, S.G.; Jung, J.Y.; Shin, J.S.; Shin, K.S.; Cho, C.W.; Rhee, Y.K.; Do Hong, H.; Lee, K.T. Immunostimulatory polysaccharide isolated from the leaves of Diospyros kaki Thumb modulate macrophage via TLR2. Int. J. Biol. Macromol. 2015, 79, 971–982.
- Shin, Y.-A.; Park, H.-R.; Hong, H.-D.; Shin, K.-S. Immuno-stimulating activities of polysaccharide fractions isolated from persimmon leaves. Korean J. Food Nutr. 2012, 25, 941–950.
- Huang, S.W.; Qiao, J.W.; Sun, X.; Gao, P.Y.; Li, L.Z.; Liu, Q.B.; Sun, B.; Wu, D.L.; Song, S.J. Secoiridoids and lignans from the leaves of Diospyros kaki Thunb. with antioxidant and neuroprotective activities. J. Funct. Foods 2016, 24, 183–195.
- Chen, G.; Xue, J.; Xu, S.X.; Zhang, R.Q. Chemical constituents of the leaves of Diospyros kaki and their cytotoxic effects. J. Asian Nat. Prod. Res. 2007, 9, 347–353.
