Equine piroplasmosis (EP), caused by the hemoparasites Theileria equi, Theileria haneyi, and Babesia caballi, is an important tick-borne disease of equines that is prevalent in most parts of the world. Infection may affect animal welfare and has economic impacts related to limitations in horse transport between endemic and non-endemic regions, reduced performance of sport horses and treatment costs.
- equine piroplasmosis
- Theileria equi
- Babesia caballi
- equine
- genotyping
Introduction
Equine piroplasmosis (EP) is a tick-borne disease of equines caused by the eukaryotic hemoparasites Theileria equi, Theileria haneyi, and Babesia caballi that has a considerable veterinary and economic impacts on the horse industry worldwide [1–5]. The parasites belong to the phylum Apicomplexa and to the order Piroplasmida [6]. EP is considered a reportable disease by the World Organization for Animal Health (OIE) (https://www.oie.int/animal-health-in-the-world/oie-listed-diseases-2020/, 15 April 2020). It is estimated that 90% of the global horse population resides in EP-endemic areas, and therefore many studies have investigated the occurrence, prevalence, risk factors, and characteristics of these parasites in different parts of the world.
Life Cycle, Vectors, and Transmission
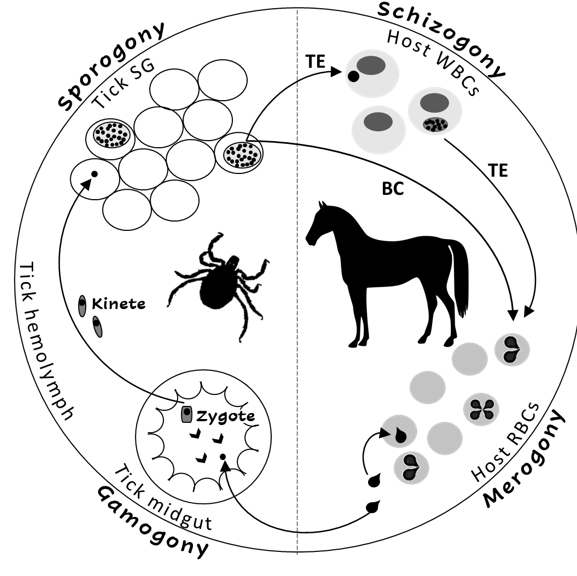
The Theileria and Babesia genera belong to the families Theileriidae and Babesiidae within the phylum Apicomplexa. The life cycles of both parasites include sexual (gamogony) and asexual (sporogony) replicative stages within the tick vector and asexual replicative stages within the equine host [2,3]. Asexual replication (merogony) in equine erythrocytes is common to both parasites, and T. equi (and likely, T. haneyi) also undergoes asexual schizogony within equine lymphocytes and monocytes prior to invasion to erythrocytes [7] (Figure 1). The term piroplasmosis derives from the pear-shaped appearance of the intra-erythrocytic stages of these parasites (merozoites). Replication in erythrocytes ultimately leads to cell rupture and the release of merozoites that invade additional cells [2,3,8].
Figure 1. The life cycle of Theileria equi (TE) and Babesia caballi (BC) in the tick vector and in the equine host. RBC—equine red blood cells, WBC—equine while blood cells, SG—tick salivary glands.
The main route of transmission to equids is by tick feeding. Over 30 species of ticks have been described as vectors of one or both T. equi and B. caballi, including the genera Hyalomma, Rhipicephalus Dermacentor, Amblyomma, and Haemaphysalis [8]. Transstadial transmission was recorded for both parasites in several tick species; however, transovarian transmission was only recorded for B. caballi [8]. Therefore, the main reservoir for T. equi is in the equine host, whilst for B. caballi it is the vector ticks [8].
Transplacental transmission in the equine host has been reported for T. equi and may lead to abortion, the birth of a sick foal with peracute neonatal EP, or the birth of unapparent carrier foal [9–19]. In some endemic areas, T. equi is considered to be a major cause of abortion [20,21]; however, the role of this parasite as a cause of abortion is not well established [22]. Iatrogenic transmission is also possible; there are several reports of infections resulting from blood transfusions, and from sharing of surgical equipment or needles [2,3,5,20]. However, these types of transmission probably do not have a major role in the epidemiology of EP.
Clinical Disease
Clinical disease in EP is mainly attributed to intravascular hemolytic anemia caused by parasite replication and damage to erythrocytes [2,3,20,23]. The clinical signs are similar following infection with both parasite species; however, clinical presentation tends to be more severe in cases of T. equi infection [2,3,20]. The incubation period ranges between 12 and 19 days for T. equi and between 10 and 30 days for B. caballi [2]. Common clinical signs are non-specific and derive from the hemolytic anemia. These include fever, inappetence, icterus, hemoglobinuria, pale mucus membranes (MM), tachycardia, and tachypnea. Thrombocytopenia has also been described. In severe cases, edema and hemorrhage might develop and may eventually lead to organ failure. Gross pathologic findings may include hepatomegaly, splenomegaly, enlarged kidneys, multifocal edema, and hemorrhages [2,3,20,23]. Anecdotal cases of EP-associated hyphema [24], cardiac arrythmias [25], and inflammatory myopathy [26] have also been reported.
Clinical manifestations following infection with either parasite species range from unapparent infection to life threatening disease. Most infected horses remain asymptomatic [2,3,20], while clinically infected horses may develop peracute, acute, subacute, or chronic disease presentations. Peracute disease is life-threatening and has been mostly described in cases of neonatal EP [2,3,20]. Acute disease is characterized by overt presentation of characteristic EP clinical signs, subacute disease manifests milder clinical signs, and chronic disease presents with non-specific signs and mild clinical pathology abnormalities [2,3,20,23,27]. The factors associated with the severity of clinical disease are unknown. Acute disease is more often observed in infections of naïve adult horses, and is less common in equine populations in endemic areas [2,3,20,23]. Stress has been suggested to induce more severe clinical signs, although the evidence to support this assumption is limited [28]. In contrast to T. equi, the newly identified T. haneyi rarely causes clinical signs, even in splenectomized horses [29].
Regardless of the initial clinical presentation, without treatment, horses infected with EP usually remain persistent subclinical (unapparent) carriers for prolonged periods of time. Carriage of T. equi is usually life-long, while B. caballi infection may be self-limiting after up to four years [2,3,20].
Immunity, Treatment, and Control
Carriage of parasites usually results in an immune response sufficient to prevent severe disease [2,3]. The precise immune mechanisms involved are not fully elucidated. Both innate and adaptive immunity appear to be necessary for parasite control, and splenectomy leads to severe clinical disease in T. equi-infected horses. Antibodies are first detected seven to 11 days after infection, and peak 30 to 54 days after infection [2,3,30–32].
The most widely used treatment for EP is imidocarb dipropionate [2,3,5]. Theileria equi is considered to be more resistant to treatment than B. caballi, and requires higher dosages and longer durations of therapy [33–36]. Two intramuscular (IM) injections 24 h apart of 2 mg/kg are recommended for the treatment of B. caballi, and four injections 72 h apart of 4 mg/kg are recommended for T. equi [33]. Although this drug is relatively safe, the latter dosage is near its 50% lethal dose (LD50), and may cause adverse signs of toxicity or even death (donkeys being more sensitive than horses) [2,3,5,35,36]. Since the administration of 4 mg/kg of imidicarb dipropionate often causes colic in horses, animals are often co-treated with flunixin meglumine or buscopan. Although complete parasite clearance is usually possible, several imidocarb diproprionate treatment cycles may be required [36]. Furthermore, imidocarb diproprionate-resistant parasites have been reported [34].
Various other chemotherapeutic agents have been reported to be potentially used against EP, with variable efficacy, mostly in vitro. Among these are anti-malaria compounds [37–39], antimicrobial agents [40–45], parasite metabolism inhibitors [34,46–53], replication inhibitors [54,55], pyrimidine synthesis inhibitors [56,57], and various plant-derived compounds [58–63]. However, most of these options have never been tested in vivo, and none is widely used.
Since no effective, commercially available vaccines against EP are yet available, control is based on a combination of drug therapy, vector control, and restricted transport of infected horses. The aims of treatment and control strategies differ between endemic and non-endemic regions. In non-endemic areas the aim is to keep the area disease-free. Thus, treatment of infected horses is aimed at complete clearance of infection, while control is mainly based on monitoring and restricting the entrance of infected horses. Several non-endemic countries, including the United States, Australia, and Japan deny entrance of seropositive horses, and either export, quarantine, or euthanize any positive animal within the country [1–3,5,20,64,65]. In addition to quarantine, horses transported from endemic to non-endemic areas usually require treatment with acaricides to prevent introduction of vector ticks (https://www.oie.int/index.php?id=169&L=0&htmfile=chapitre_equine_piroplasmosis.htm). In endemic areas, unapparent carriage and the development of premonition are usually encouraged, rather than parasite clearance, to prevent clinical outbreaks. Thus, treatment is usually aimed only to reduce clinical sings of acute infection, while strategic use of acaricides is recommended to reduce, but not eliminate, exposure to ticks [1–3,5,20].
Diagnosis
Diagnosis of EP infection is important to identify unapparent carriers, especially prior to transport into non-endemic areas, and to identify EP as a cause of disease clinically ill animals, especially due to the non-specific nature of clinical signs in EP infection. Various diagnostic techniques have been reported based on clinical signs, microscopic examination, culture, serology, and molecular assays [2,3,5,66].
Traditionally, identification of piriform parasites in Giemsa-stained blood smears was the diagnostic method of choice in clinical cases [2,3,5,66]. However, the sensitivity of this method is low, leading to false negative results in many chronic and subclinical cases, when parasite loads are low. In vitro culture methods proved more sensitive and specific; however, these methods are time-consuming,require fresh blood samples and skilled personnel, and therefore are not frequently used as routine diagnostic tests [2,3,5,20,66–71].
Serological diagnosis has better sensitivity and specificity for the detection of unapparent carrier horses; however, these assays do not provide information on current parasite load for interpretation of clinical disease states. Several EP-specific serologic assays, comprised of various methods, including a complement fixation test (CFT) [72,73], an indirect immunoflorescent antibody test (IFAT) [72,73], and an enzyme-linked immunosorbent assay (ELISA) [74–77], have been developed. The CFT is very specific; however, it may give false negative results, especially after treatment and with chronicity, since IgG(T) is not complement-fixing. IFAT is more sensitive than the CFT and remains positive in chronic cases; however, interpretation of the results is subjective and difficult to standardize [2,3,5,20,68,72,73]. Different ELISA tests were developed to detect EP infection, including an indirect ELISA (iELISA) [74] and a competitive ELISA (cELISA) [78]. To improve the standardization and performance of these tests, several cELISA assays were developed using purified recombinant antigens, and are currently the United States Department of Agriculture (USDA) and OIE recommended tests for international horse transport screening. The use of a single epitope also reduces the chance of cross-reactivity between the parasites. The immunodominant T. equi surface antigens equine merozoite antigen (ema)-1 and ema-2, and the B. caballi rhoptry-associated protein (rap)-1 were successfully used and proven superior to IFAT and CFT in several studies [2,3,5,20,30,68,74,76,77,79–81]. Nevertheless, some heterogeneity has been recorded between isolates, and the USDA-approved B. caballi rap-1 cELISA assay did not detect infected horses in South-Africa and in the Middle East [82–84].
Molecular diagnosis, based on the detection of parasite DNA in equine blood by polymerase chain reaction (PCR), is gaining popularity for the detection of parasites in both clinical and carrier animals. These methods are more sensitive than microscopic examination, and more clinically useful than serology, since they represent current infection. These methods can also be designed to distinguish between parasite species or genotypes. Currently, these methods are more often used for research than in clinical practice [2,3,5,20,68,85–92]. Numerous assays targeting one or multiple EP parasites, including conventional PCR [86,87], nested PCR (nPCR) [90,93], real-time PCR (rtPCR) [82,94–99], multiplex PCR (mPCR) [87,89], reverse line blot (RLB) [100,101], and loop mediated isothermal amplification (LAMP) [85,88,91], have been developed. Several of these assays were determined to have high sensitivity, with a detection limit of 10−7% parasitized erythrocytes (PE) [2,3,5,20,68,85–92]. Quantitative methods, such as rtPCR (qPCR), have also been developed, but are mostly applied to increase the sensitivity of parasite detection, and are rarely used to evaluate parasite loads [94–99,102].
Epidemiology
The transmission dynamics of T. equi and B. caballi are different. In endemic areas, animals are usually exposed at a young age to both parasites and develop premonition. Carriage of T. equi is usually life-long; thus, the observed prevalence increases with age and the host is the main reservoir of parasites. The prevalence of B. caballi, on the other hand, does not increase with age and is higher in younger animals. Clearance of B. caballi is possible, and the parasite is transovarially transmitted by ticks, suggesting the main reservoir of this parasite is the tick [2,3,5,8,20,113,123–126].
EP is endemic in most parts of the world where competent tick vectors are present. Few countries are considered non-endemic, including the US and Canada, the United Kingdom (UK) and Ireland, Northern Europe, Iceland and Greenland, Singapore, Japan, New Zealand, and Australia. In some of these countries, EP has been reported, but is limited to specific areas and is not widespread or endemic [2,3,5,8,123].
The only risk factors consistently associated with EP infection are management practices and tick exposure. Other factors, including host species, breed, age, sex, and activity, have been inconsistently associated with infection (reviewed in: [5,123]). Although most EP-endemic areas are within tropical and temperate regions, recent global warming and increased global transportation have led to the spread of both parasites and vectors to previously non-endemic areas, such as the UK [127]. Some of these areas are suitable habitats for potential vector ticks and are therefore susceptible to epizootic disease spread—hence the significance of OIE monitoring of the distribution and spread of EP, a summary of which is available through the OIE’s new World Animal Health Information Database (WAHIS) (https://www.oie.int/wahis_2/public/wahid.php/Wahidhome/Home, 15 April 2020).
Clinical manifestation of EP is less common in endemic areas, since early exposure is likely to induce protection [2,3,20,23]. However, clinical cases have been reported in resident horses in both non-endemic (Poland, The Netherlands, USA) [204–207] and in endemic areas (Israel, Italy, Romania, Spain) [25,107,208–210]. This highlights the fact that in areas which are considered endemic, there are sub-populations of horses that differ in their exposure to vector ticks, and subsequently to infection with EP and to the development of premonition. These sub-populations should be approached differently in application of preventive measures and treatment to reduce the chance of clinical disease [1–3,5,20,125].
Both parasites are endemic in similar areas, although T. equi is more frequently reported and is usually more prevalent than B. caballi. This may reflect the different transmission cycles of these parasites, since the main reservoirs of B. caballi and T. equi are in ticks and horses, respectively [2,3,8,20,211]. In addition, parasite loads in both clinically affected horses and in unapparent carriers are usually higher in cases of T. equi infection than in B. caballi infection, increasing the odds it will be detected by a diagnostic test [2,3,209].
In addition to horses, both parasites have been reported in other equids including domestic donkeys [109,137,138,181,212–219], wild donkeys [214,220], mules [109,138,181,212,216], and zebras [102,214,220–225]; and in non-equids, including dogs [145,226–230], camels [231,232], cattle [233], and a tapir [234] (recently reviewed in: [5,214]). Donkeys are considered more resistant to infection than horses [217]; however, this assumption is not well established, since the data regarding domestic equids (donkeys and mules) is less comprehensive than in horses, and many surveys use a population of different equine species. Reports in other animals are anecdotal, and the role of other species as a reservoir of EP has not been demonstrated. All reports describing EP in other animals originate from endemic areas of EP in horses.
Genotyping
Since their discovery in 1901 [235], the taxonomy of these parasites has been challenged, and they have been re-named and re-classified several times. Currently, B. caballi is considred a “true Babesia,” while T. equi (formerly: B. equi) has been classified as Theileria based on its extra-erythrocytic life stage in lymphocytes and the absence of transovarian transmission in ticks [211]. Molecular investigations indicate that it possesses characteristics of both Babesia and Theileria, and is possibly placed between the two [6,236–239]. The full genome of T. equi was constructed from an American strain, which had been used in numerous phylogenetic studies evaluating the genetic diversity between and within piroplasm species [238]. Considerable genetic variation has been found within T. equi, and recent discoveries of novel, closely related, species, including T. haneyi, suggest that current classification of T. equi may include several distinct organisms [4,140].
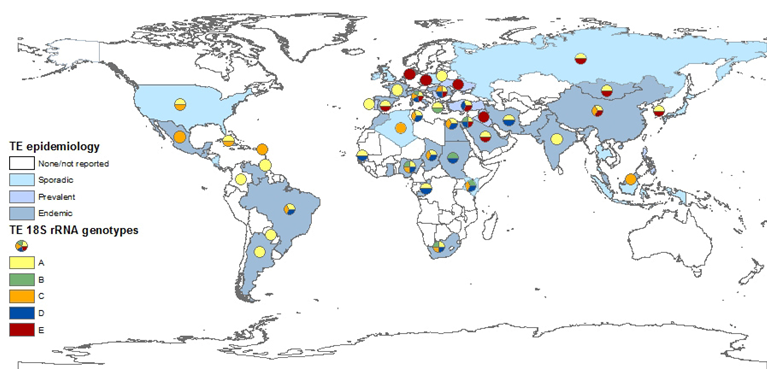
Phylogenetic studies investigating inter-species diversity between piroplasms have mainly focused on the 18S rRNA [6,140,179,236,239–240], β-tubulin [241], mitochondrial genes [237], and ema-1 genes [242,243], while intra-species diversity and genotyping mainly focused on the 18S rRNA [17,83,100,102,107,112,140,144,209,244,245], T. equi ema-1 [95,107,112,130,209,245], and B. caballi rap-1 [82–84,107,112,130,209] genes. Five T. equi 18S rRNA genotypes (A–E) have been identified up to date [89,100,102,163,246,247], and three (A–C) ema-1 genotypes [95]. Three B. caballi 18S rRNA genotypes have been described (A, B1, and B2) [94,100,209], along with three rap-1 genotypes (A1, A2, B) [82–84]. Recently, the identification of T. equi by its 18S rRNA gene has been scrutinized following the discovery of several new species that were indistinguishable from T. equi based on this locus, suggesting this classification may actually represent several distinct species [4,140]. No specific genotype had been found to be linked to increased virulence; however, two unrelated studies reported a higher frequency of clinically affected horses are infected with T. equi 18S rRNA genotype A [107,209].
Despite concerns raised that T. equi 18S rRNA gene may not allow clear distinction between closely related Theileria species [4,140], the analysis of its various sequences in the GenBank database provided the best basis to determine T. equi genotype distribution (Figure 2), while the ema-1 and ema-2 loci are more conserved. The most widely distributed T. equi 18S rRNA genotype is genotype A. This genotype has been isolated in most countries and on all continents. This genotype is also the only one that has been fully sequenced. Although there is no concrete evidence linking any specific genotype to parasite virulence, at least two studies suggest infection with genotype A leads to more severe clinical disease [107,209], and this correlation has also been described during several outbreaks [244]. Moreover, this is the main genotype isolated from ticks [254] and the only genotype isolated from dogs. Genotype C is also widely distributed, and was also found on all continents. In the Americas, genotypes A and C are the predominant genotypes. Genotype D was mainly found in Africa, the Mediterranean region, and the Middle East, and had not been isolated from Northern Europe, the Far East, North and Central America, or the Caribbean region. Genotype E, on the other hand, is mainly found in the Far East, Northern and Eastern Europe and the Middle East, but not in Africa, America or the Caribbean. Genotype B was only detected in Africa and the Mediterranean region. The differences in the distribution of each genotype is important in understanding the spread of parasites and the infection dynamics within and between equine populations. Recent studies showed that in endemic areas, many horses are co-infected with several genotypes of T. equi, and that the predominant genotype or genotypes differ between equine hosts and subpopulations [102,125,221,255]. Co-infection is also possible with other related species, including T. haneyi [28] and B. caballi (Table 1). The significance of this co-infection and the relations between parasites or genotypes within the host should be further investigated, since it is likely to be a part of maintaining the enzootic stability in endemic areas.
Recent work demonstrated a correlation between B. caballi 18S rRNA and rap-1 genotypes [209], making the classification more robust. However, since relatively fewer studies provided genotypic characterization of B. caballi, additional molecular data from various locations should be gathered and analyzed in order to understand the global molecular epidemiology of this parasite.
Figure 2. Global prevalence of T. equi, and the distribution of T. equi 18S rRNA genotypes. The map was constructed based on epidemiological data published in the last 20 years (2000–2019). Endemic: over 30%, prevalent: 10–29%, sporadic: under 10% or singular outbreaks. Genotyping was performed on all sequences submitted to GenBank and classification was based on previously reported clades.
Conclusion
EP is endemic in most parts of the world, and is spreading further into more temperate climate zones previously considered parasite-free. Basing the diagnosis on advanced molecular tools and increasing the understanding of the differences between genotypes will enable to better control these important pathogens, thus, reducing their clinical and economic impact.
This entry is adapted from the peer-reviewed paper 10.3390/pathogens9110926