Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Medicine, Research & Experimental
Adipose tissue (AT) dysregulation is a key process in the pathophysiology of obesity and its cardiometabolic complications. Mitochondria are cytoplasmic organelles that play a critical role in the energy metabolism of all eukaryotic cells and generate energy in the form of adenosine triphosphate (ATP).
- obesity
- gut microbiota
- mitochondria
- white adipose tissue
- crosstalk
1. White and Brown Adipose Tissue
Historically, AT has been considered as an inert connective tissue with only cushioning and thermal insulation functions, but in recent years it has acquired the status of an endocrine organ because of its important effects on the regulation of metabolism through the secretion of hormones (such as leptin and adiponectin) and cytokines (also called “adipokines”) [1].
Adipocytes are those cells primarily characterizing AT, which nonetheless, is also composed of many other cell types, such as pre-adipocytes, fibroblasts, vascular endothelial cells, and immune cells [2].
Altogether, we can distinguish two main types of adipose tissue that differ in cell structure, histology, amount, anatomic location, and function: white adipose tissue (WAT) and brown adipose tissue (BAT) (Figure 1).
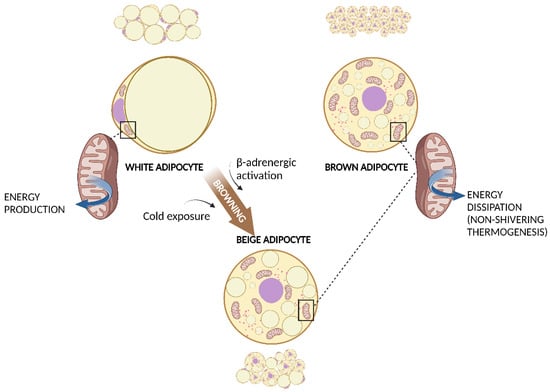
Figure 1. WAT, BAT, and WAT browning. White adipocyte has one large droplet in the centre of the cell that compresses nucleus and mitochondria at one pole. Brown adipocyte has multiple small lipid droplets and more mitochondria, spread out between the droplets. Beige adipocyte has intermediate characteristics. Cold exposure and β-adrenergic activation determine the browning of WAT. Both brown and beige mitochondria are involved in non-shivering thermogenesis. Created with BioRender.com (accessed on 13 March 2023). Abbreviations: BAT, brown adipose tissue; WAT, white adipose tissue.
WAT is composed of white adipocytes: large cells with a diameter of 20–150 μm, almost completely occupied by a single, large lipid droplet that pushes the nucleus and other organelles to one pole of the cell. Brown adipocytes are smaller cells with a diameter of about 10–25 μm and many lipid droplets that give the cell a multilocular aspect. Another important characteristic of brown adipocytes is the higher number of mitochondria, which determines the typical brown coloration of BAT under microscopy [3].
WAT is more abundant than BAT in humans. There are two main anatomic districts where WAT can be found and where it assumes different functions: the subcutaneous district (subcutaneous adipose tissue, SAT) and the visceral district (visceral adipose tissue, VAT) [4]. SAT is localized just beneath the skin and represents about the 85% of total body fat in a normal weight adult. It can be found principally in abdominal, gluteal, and femoral depots, with differences influenced also by the gender (in women, it is more abundant in the gluteal–femoral region) [5]. Other than having a mechanic protective function acting as a cushion against external traumatic and thermal stress, SAT plays an important role in glucose and lipid homeostasis [6]. VAT is composed of the fat deposits in the abdominal cavity: omental (hanging from the stomach), mesenteric (along the intestine) and epiploic (near the colon) fat. Typically accounting for less than 20% of the total body fat, VAT expansion is associated with the development of the metabolic complications of obesity and a substantial increase in cardiovascular risk [7]. Waist circumference can be considered to be a valid estimator of VAT accumulation and denotes a more robust independent risk factor for insulin resistance, T2D, dyslipidemia, and atherosclerosis than body mass index (BMI) [8][9].
In addition to SAT and VAT, there are ectopic deposits of fat that infiltrate other anatomic structures such as skeletal muscle, arteries, and myocardium, which despite their smaller amounts play a significant role in the development of obesity complications. Indeed, intermuscular fat has been linked to insulin resistance and muscle catabolism, whereas the epicardial AT surrounding the myocardium and coronary arteries plays a role in the pathogenesis of coronary atherosclerosis and atrial arrhythmias [10][11].
Unlike other mammals such as small rodents, in humans BAT represents a minimum percentage of body weight. BAT volume can be assessed with 18 fluoro-deoxy-glucose positron emission tomography/computed tomography (18FDG-PET/CT) [12]. There is a higher amount in infants (when skeletal muscles have not developed enough to maintain body temperature after cold exposure through shivering thermogenesis) and the amount tends to decrease thereafter in adulthood, remaining in the supraclavicular region and in small quantities around the great vessels in the paravertebral and mediastinal areas [13].
The varying distribution and differences in morphology and histology between WAT and BAT reflect their different functions. WAT plays an important role in energy homeostasis, as it is able to store surplus energy in the form of triglycerides that can be then mobilized during periods of high energy demand [14]. In presence of a chronically positive energy balance, WAT can expand following two main pathways: adipocyte hyperplasia (increase in the number of adipocytes thanks to the proliferation and differentiation of mesenchymal stem cells) and hypertrophy (enlargement of existing adipocytes due to increased lipid storage). Although hyperplasia has been associated with a more metabolically healthy condition, adipocyte hypertrophy is accompanied by impaired AT angiogenesis, fibrosis, oxidative stress, and inflammatory cell proliferation that lead to low-grade chronic inflammation and insulin resistance [15], two well-known complications of obesity that contribute to increasing cardiovascular risk [16].
WAT also exerts an endocrine function, as it produces enzymes involved in steroid hormone metabolism (e.g., aromatase) and secretes adipokines which control energy, lipid, and carbohydrate metabolism and which can modulate immune system activity not only locally, but at the systemic level [17]. Leptin is a peptide hormone synthesized by adipose cells in response to food intake and provides the hypothalamus with information to control feeding and regulate body weight homeostasis. Leptin circulation levels are proportional to body fat mass and reflect the body’s energy reserves. Increased leptin levels induce anorexigenic factors (such as cocaine–amphetamine-related transcript) and suppress orexigenic neuropeptides (such as neuropeptide Y), thereby reducing food intake [18]. On the contrary, adiponectin is secreted in higher quantities when there is a reduction in body fat, reflecting a chronically negative energy balance, and is associated with anti-inflammatory and insulin-sensitizing effects characteristic of weight loss [19].
As well as WAT, BAT is also involved in energy homeostasis, but its role is diametrically opposite: expending energy instead of storing it. BAT is the site of the so-called non-shivering thermogenesis. Different from shivering thermogenesis produced by the involuntary contraction of muscles caused by cold exposure, non-shivering thermogenesis is carried out by BAT mitochondria via the uncoupling protein-1 (UCP-1) [20]. Brown adipocytes present a large number of small lipid droplets which are more easily accessible for hydrolysis and oxidation of free fatty acids (FFAs). UCP-1, expressed within the inner membrane of mitochondria, uncouples oxidation from subsequent adenosine diphosphate phosphorylation and thereby energy is dissipated as heat. Cold exposure is the principal activator of BAT and the effect of cold is mediated via the activated sympathetic nervous system. Interestingly, chronic cold exposure causes an increase in UCP-1 expression and mitochondrial content in white adipocytes in a process known as browning (Figure 1). These adipocytes have characteristics that are in between those of white and brown adipocytes and are called “brite” or beige adipocytes, and they can produce heat with UCP-1, similarly to BAT [21]. Chronic exercise has also been associated with subcutaneous WAT browning [22]. As BAT activation has been associated with a healthier inflammatory profile and a reduction in insulin-resistance, WAT browning is considered an attractive strategy for the prevention of metabolic diseases [23].
2. Mitochondria in WAT and BAT
Mitochondria are cytoplasmic organelles that play a critical role in the energy metabolism of all eukaryotic cells and generate energy in the form of adenosine triphosphate (ATP). There are two principal ways of producing ATP in mitochondria: (1) oxidation of carbohydrates, fats, and proteins through the tricarboxylic acid (TCA) cycle and (2) β-oxidation of fatty acids [24]. The mitochondria play a central role in all cellular processes by catalyzing the oxidation of fuel molecules (glucose, fat acid, and amino acids) and transforming the electrons to molecular oxygen with concomitant energy transduction into ATP (oxidative phosphorylation, and OXPHOS) [25]. The OXPHOS includes five enzymatic complexes and two mobile electron carriers (NADH and FADH2) that work in a mitochondrial respiratory chain (I: NADH–coenzyme Q reductase; II: succinate–coenzyme Q reductase; III: coenzyme QH2 cytochrome-c reductase; IV: cytochrome-c oxidase; V: ATP synthase) [26].
Mitochondria are surrounded by a double-membrane system of inner and outer membranes. The inner membrane is folded in cristae that increase the overall surface where OXPHOS takes place under aerobic conditions [24]. A unique characteristic of mitochondria is the presence of a proper genome, a circular mitochondrial DNA (mtDNA) that mainly encodes for proteins related to OXPHOS which are added to over one thousand other mitochondrial proteins encoded by nuclear DNA (nDNA) [27]. Mitochondria are very dynamic organelles capable of meeting bioenergy or oxidative challenges by changing their morphology and protein content. Through fusion, mitochondria can exchange proteins and mtDNA to improve their function, then they can divide again by fission, producing newly restored mitochondria [28]. Aged and damaged mitochondria which can no longer be restored are selectively degraded by lysosomes in a process called mitophagy [29]. Impairment to these processes and overall mitochondrial homeostasis can lead to the development of metabolic diseases.
In WAT, adipocytes contain a relatively low mitochondrial mass per overall cell size compared with other cell types [30], but mitochondria exert a fundamental role in regulating lipid turnover. In presence of a positive energy balance, mitochondria generate the intermediary metabolites needed for lipogenesis, such as acetyl-CoA for fatty acid synthesis and esterification with glycerol-3-phosphate into triglycerides. Then, during fasting or in conditions of high energy demand, adipocytes release FFAs via lipolysis, thanks to hormone-sensitive lipase and adipose triglyceride lipase [31].
Mitochondria are also involved in adipocyte differentiation and adipogenesis [32]. During adipogenesis, mitochondrial oxygen consumption and the amount of mitochondrial proteins increase [33]. Mitochondrial biogenesis shares many key regulators with adipogenesis, such as the peroxisome proliferator-activated receptor gamma (PPARγ) and the peroxisome proliferator-activated receptor gamma coactivator-1alpha (PGC-1α), with which it co-activates [34]. Activation of PPARγ nuclear receptors prompts the transcription of genes related to a wide spectrum of biological functions, from mitochondrial and turnover to antioxidant defense and immune response. Rosiglitazone, a PPARγ agonist originally developed for the treatment of T2D, promotes mitochondrial biogenesis and remodeling in WAT that improve the entire body’s energy metabolism and insulin sensitivity [35]. PGC-1α is a key component of mitochondrial biogenesis and induced by reactive oxygen species (ROS) which also regulate adipogenesis, as ROS are required for the differentiation of preadipocytes to adipocytes. Imbalance in ROS levels triggers mitochondrial and adipocyte dysfunction resulting in WAT oxidative stress and inflammation [36].
Moreover, mitochondria may also be involved in WAT adipokine secretion. Adiponectin and mitochondrial biogenesis are interconnected, as one stimulates the other. It has been demonstrated that adiponectin inhibits mitochondrial mediated apoptosis and, in turn, mitochondrial biogenesis positively regulates adiponectin secretion, in a virtuous circle which improves glucose metabolism and insulin sensitivity [37]. On the contrary, impaired mitochondrial function in WAT may explain the reduced adiponectin levels observed in obesity associated with low-grade chronic inflammation and cardiovascular diseases [38]. With regard to leptin, it has been reported that mitochondrial function, rather than mitochondrial biogenesis or mass, can be improved by administering leptin [39]; however, further studies are required to determine whether there is a direct relationship with AT mitochondria.
Most of our knowledge about the metabolism and function of mitochondria in WAT comes from studies on SAT. Less is known about VAT mitochondria, particularly in conditions of good health. In rats, VAT presents higher mitochondrial density, mtDNA content, mitochondrial enzymes, and respiration as compared to SAT [40]. In obesity, mitochondrial respiration and phosphorylation activity seem significantly lower in VAT, supporting the hypothesis of a dysfunctional activity of VAT mitochondria [41].
We previously reported differences in WAT and BAT morphology, histology, distribution, and function, but BAT also shows specific mitochondrial characteristics (Table 1). First of all, BAT appears brownish in microscopic images just because of the higher expression of cytochrome oxidase, an iron-containing heme cofactor, that indicates higher mitochondrial concentrations [32]. Transmission electron microscopy images from rat interscapular BAT show that mitochondria in BAT are bigger, have a spherical shape, and contain more packed cristae [42]. Their distinctive feature, both in rodents and in humans, is the higher expression of UCP-1 [43] which reflects their main function of heat generation through non-shivering thermogenesis [44].
Table 1. WAT and BAT mitochondria.
| Mitochondrial Characteristics | WAT | BAT |
|---|---|---|
| Content | Lower | Higher |
| Dimension * | Smaller | Bigger |
| Shape * | Elongated | Spherical |
| Inner membrane cristae * | Less packed | More packed |
| UCP-1 expression | Lower | Higher |
| Main function | Energy storage and lipid homeostasis | Non-shivering thermogenesis |
Abbreviations: BAT, brown adipose tissue; UCP-1, uncoupling protein-1; WAT, white adipose tissue. * studies on rats.
In brown adipocytes that are highly vascularized and innervated cells, mitochondria are immersed amongst numerous small lipid droplets [45]. Cold induces sympathetic stimulation of brown adipocytes via β3-adrenergic receptors in rodents but predominantly by β1-adrenergic receptors in humans [46]. The β-adrenergic receptor couples with a Gs protein which activates adenylyl cyclase, leading to the formation of cAMP as a secondary messenger. Subsequently, cAMP promotes the activation of cytosolic lipolytic enzymes (hormone-sensitive lipase, adipose triglyceride lipase, and perilipin) and lipolysis produces FFAs [47]. Through the carnitine cycle, FFAs are converted into long chain acyl-carnitine esters that are imported into the mitochondrial matrix and then converted back into acylCoA, serving as a substrate for the TCA cycle [48]. Meanwhile, FFAs directly activate UCP-1, which dissipates the electrochemical proton gradient produced by the respiratory chain, thus catalysing the leak of protons across the mitochondrial inner membrane. In this way, energy from the oxidation of respiratory substrates that would otherwise be utilized for ATP synthesis is released as heat [49].
This entry is adapted from the peer-reviewed paper 10.3390/nu15071723
References
- Colleluori, G.; Perugini, J.; Giordano, A.; Cinti, S. From Obesity to Diabetes: The Role of the Adipose Organ. Handb. Exp. Pharmacol. 2022, 274, 75–92.
- Corvera, S. Cellular Heterogeneity in Adipose Tissues. Annu. Rev. Physiol. 2021, 83, 257–278.
- Cinti, S. Adipose Organ Development and Remodeling. Compr. Physiol. 2018, 8, 1357–1431.
- Smith, S.R.; Lovejoy, J.C.; Greenway, F.; Ryan, D.; deJonge, L.; de la Bretonne, J.; Volafova, J.; Bray, G.A. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metabolism 2001, 50, 425–435.
- Geer, E.B.; Shen, W. Gender differences in insulin resistance, body composition, and energy balance. Gend. Med. 2009, 6 (Suppl. S1), 60–75.
- Caton, P.W.; Evans, E.A.; Philpott, M.P.; Hannen, R.F. Can the skin make you fat? A role for the skin in regulating adipose tissue function and whole-body glucose and lipid homeostasis. Curr. Opin. Pharmacol. 2017, 37, 59–64.
- Guglielmi, V.; Sbraccia, P. Obesity phenotypes: Depot-differences in adipose tissue and their clinical implications. Eat Weight Disord. 2018, 23, 3–14.
- Zhang, C.; Rexrode, K.M.; van Dam, R.M.; Li, T.Y.; Hu, F.B. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality: Sixteen years of follow-up in US women. Circulation 2008, 117, 1658–1667.
- Sbraccia, P.; D’Adamo, M.; Guglielmi, V. Is type 2 diabetes an adiposity-based metabolic disease? From the origin of insulin resistance to the concept of dysfunctional adipose tissue. Eat Weight Disord. 2021, 26, 2429–2441.
- Guglielmi, V.; Sbraccia, P. Epicardial adipose tissue: At the heart of the obesity complications. Acta Diabetol. 2017, 54, 805–812.
- Venteclef, N.; Guglielmi, V.; Balse, E.; Gaborit, B.; Cotillard, A.; Atassi, F.; Amour, J.; Leprince, P.; Dutour, A.; Clément, K.; et al. Human epicardial adipose tissue induces fibrosis of the atrial myocardium through the secretion of adipo-fibrokines. Eur. Heart J. 2015, 36, 795–805a.
- Maliszewska, K.; Kretowski, A. Brown Adipose Tissue and Its Role in Insulin and Glucose Homeostasis. Int. J. Mol. Sci. 2021, 22, 1530.
- Cypess, A.M.; Lehman, S.; Williams, G.; Tal, I.; Rodman, D.; Goldfine, A.B.; Kuo, F.C.; Palmer, E.L.; Tseng, Y.H.; Doria, A.; et al. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 2009, 360, 1509–1517.
- Rosen, E.D.; Spiegelman, B.M. Adipocytes as regulators of energy balance and glucose homeostasis. Nature 2006, 444, 847–853.
- Guglielmi, V.; Cardellini, M.; Cinti, F.; Corgosinho, F.; Cardolini, I.; D’Adamo, M.; Zingaretti, M.C.; Bellia, A.; Lauro, D.; Gentileschi, P.; et al. Omental adipose tissue fibrosis and insulin resistance in severe obesity. Nutr. Diabetes 2015, 5, e175.
- Crewe, C.; An, Y.A.; Scherer, P.E. The ominous triad of adipose tissue dysfunction: Inflammation, fibrosis, and impaired angiogenesis. J. Clin. Investig. 2017, 127, 74–82.
- Kershaw, E.E.; Flier, J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556.
- Flier, J.S. The adipocyte: Storage depot or node on the energy information superhighway? Cell 1995, 80, 15–18.
- Barbarroja, N.; Lopez-Pedrera, C.; Garrido-Sanchez, L.; Mayas, M.D.; Oliva-Olivera, W.; Bernal-Lopez, M.R.; El Bekay, R.; Tinahones, F.J. Progression from high insulin resistance to type 2 diabetes does not entail additional visceral adipose tissue inflammation. PLoS ONE 2012, 7, e48155.
- Giralt, M.; Villarroya, F. White, brown, beige/brite: Different adipose cells for different functions? Endocrinology 2013, 154, 2992–3000.
- Shabalina, I.G.; Petrovic, N.; de Jong, J.M.; Kalinovich, A.V.; Cannon, B.; Nedergaard, J. UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell Rep. 2013, 5, 1196–1203.
- De Matteis, R.; Lucertini, F.; Guescini, M.; Polidori, E.; Zeppa, S.; Stocchi, V.; Cinti, S.; Cuppini, R. Exercise as a new physiological stimulus for brown adipose tissue activity. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 582–590.
- Georgiev, A.; Granata, C.; Roden, M. The role of mitochondria in the pathophysiology and treatment of common metabolic diseases in humans. Am. J. Physiol. Cell Physiol. 2022, 322, C1248–C1259.
- Goldenthal, M.J.; Marín-García, J. Mitochondrial signaling pathways: A receiver/integrator organelle. Mol. Cell Biochem. 2004, 262, 1–16.
- Kakkar, P.; Singh, B.K. Mitochondria: A hub of redox activities and cellular distress control. Mol. Cell Biochem. 2007, 305, 235–253.
- Tang, J.X.; Thompson, K.; Taylor, R.W.; Oláhová, M. Mitochondrial OXPHOS Biogenesis: Co-Regulation of Protein Synthesis, Import, and Assembly Pathways. Int. J. Mol. Sci. 2020, 21, 3820.
- Pagliarini, D.J.; Calvo, S.E.; Chang, B.; Sheth, S.A.; Vafai, S.B.; Ong, S.E.; Walford, G.A.; Sugiana, C.; Boneh, A.; Chen, W.K.; et al. A mitochondrial protein compendium elucidates complex I disease biology. Cell 2008, 134, 112–123.
- Youle, R.J.; van der Bliek, A.M. Mitochondrial fission, fusion, and stress. Science 2012, 337, 1062–1065.
- Ma, K.; Chen, G.; Li, W.; Kepp, O.; Zhu, Y.; Chen, Q. Mitophagy, Mitochondrial Homeostasis, and Cell Fate. Front. Cell Dev. Biol. 2020, 8, 467.
- Kusminski, C.M.; Scherer, P.E. Mitochondrial dysfunction in white adipose tissue. Trends Endocrinol. Metab. 2012, 23, 435–443.
- Heinonen, S.; Jokinen, R.; Rissanen, A.; Pietiläinen, K.H. White adipose tissue mitochondrial metabolism in health and in obesity. Obes. Rev. 2020, 21, e12958.
- De Pauw, A.; Tejerina, S.; Raes, M.; Keijer, J.; Arnould, T. Mitochondrial (dys)function in adipocyte (de)differentiation and systemic metabolic alterations. Am. J. Pathol. 2009, 175, 927–939.
- Wilson-Fritch, L.; Burkart, A.; Bell, G.; Mendelson, K.; Leszyk, J.; Nicoloro, S.; Czech, M.; Corvera, S. Mitochondrial biogenesis and remodeling during adipogenesis and in response to the insulin sensitizer rosiglitazone. Mol. Cell Biol. 2003, 23, 1085–1094.
- Rosen, E.D.; Spiegelman, B.M. Molecular regulation of adipogenesis. Annu. Rev. Cell Dev. Biol. 2000, 16, 145–171.
- Wilson-Fritch, L.; Nicoloro, S.; Chouinard, M.; Lazar, M.A.; Chui, P.C.; Leszyk, J.; Straubhaar, J.; Czech, M.P.; Corvera, S. Mitochondrial remodeling in adipose tissue associated with obesity and treatment with rosiglitazone. J. Clin. Investig. 2004, 114, 1281–1289.
- Castro, J.P.; Grune, T.; Speckmann, B. The two faces of reactive oxygen species (ROS) in adipocyte function and dysfunction. Biol. Chem. 2016, 397, 709–724.
- Koh, E.H.; Park, J.Y.; Park, H.S.; Jeon, M.J.; Ryu, J.W.; Kim, M.; Kim, S.Y.; Kim, M.S.; Kim, S.W.; Park, I.S.; et al. Essential role of mitochondrial function in adiponectin synthesis in adipocytes. Diabetes 2007, 56, 2973–2981.
- Nakajima, T.; Yokota, T.; Shingu, Y.; Yamada, A.; Iba, Y.; Ujihira, K.; Wakasa, S.; Ooka, T.; Takada, S.; Shirakawa, R.; et al. Impaired mitochondrial oxidative phosphorylation capacity in epicardial adipose tissue is associated with decreased concentration of adiponectin and severity of coronary atherosclerosis. Sci. Rep. 2019, 9, 3535.
- Blanquer-Rosselló, M.M.; Santandreu, F.M.; Oliver, J.; Roca, P.; Valle, A. Leptin Modulates Mitochondrial Function, Dynamics and Biogenesis in MCF-7 Cells. J. Cell Biochem. 2015, 116, 2039–2048.
- Deveaud, C.; Beauvoit, B.; Salin, B.; Schaeffer, J.; Rigoulet, M. Regional differences in oxidative capacity of rat white adipose tissue are linked to the mitochondrial content of mature adipocytes. Mol. Cell Biochem. 2004, 267, 157–166.
- Lindinger, A.; Peterli, R.; Peters, T.; Kern, B.; von Flüe, M.; Calame, M.; Hoch, M.; Eberle, A.N.; Lindinger, P.W. Mitochondrial DNA content in human omental adipose tissue. Obes. Surg. 2010, 20, 84–92.
- Cinti, S. Transdifferentiation properties of adipocytes in the adipose organ. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E977–E986.
- Carpentier, A.C.; Blondin, D.P.; Virtanen, K.A.; Richard, D.; Haman, F.; Turcotte, É.E. Brown Adipose Tissue Energy Metabolism in Humans. Front. Endocrinol. 2018, 9, 447.
- Forner, F.; Kumar, C.; Luber, C.A.; Fromme, T.; Klingenspor, M.; Mann, M. Proteome differences between brown and white fat mitochondria reveal specialized metabolic functions. Cell Metab. 2009, 10, 324–335.
- Cannon, B.; Nedergaard, J. Brown adipose tissue: Function and physiological significance. Physiol. Rev. 2004, 84, 277–359.
- Nedergaard, J.; Cannon, B. The changed metabolic world with human brown adipose tissue: Therapeutic visions. Cell Metab. 2010, 11, 268–272.
- Chaves, V.E.; Frasson, D.; Kawashita, N.H. Several agents and pathways regulate lipolysis in adipocytes. Biochimie 2011, 93, 1631–1640.
- Knottnerus, S.J.G.; Bleeker, J.C.; Wüst, R.C.I.; Ferdinandusse, S.; IJlst, L.; Wijburg, F.A.; Wanders, R.J.A.; Visser, G.; Houtkooper, R.H. Disorders of mitochondrial long-chain fatty acid oxidation and the carnitine shuttle. Rev. Endocr. Metab. Disord. 2018, 19, 93–106.
- Crichton, P.G.; Lee, Y.; Kunji, E.R. The molecular features of uncoupling protein 1 support a conventional mitochondrial carrier-like mechanism. Biochimie 2017, 134, 35–50.
This entry is offline, you can click here to edit this entry!
