Additive manufacturing facilitates the design of porous metal implants with detailed internal architecture. A rationally designed porous structure can provide to biocompatible titanium alloys biomimetic mechanical and biological properties for bone regeneration. However, increased porosity results in decreased material strength. The porosity and pore sizes that are ideal for porous implants are still controversial in the literature, complicating the justification of a design decision. Recently, metallic porous biomaterials have been proposed for load-bearing applications beyond surface coatings. This recent science lacks standards, but the Quality by Design (QbD) system can assist the design process in a systematic way.
- porous implants
- bone implants
- metamaterials
- titanium
- mechanical properties
- pore size
- unit cell
- porosity
- elastic modulus
- compressive strength
- additive manufacturing
1. Current Issues with Traditional Bone Implants and Scaffolds
Many physical conditions necessitate bone tissue replacements and joint implants. Some of these conditions are caused by degenerative diseases, birth defects, and orthopaedic traumas [1]. However, despite the tremendous progress in biomedical engineering, 20% of patients subjected to joint reconstructive surgery experience significant problems [2]. This situation is reflected in the fact that orthopaedic products, such as knee and hip prostheses, are the fifth most recalled medical products; of these recalls, 48% are due to manufacturing issues and 34% to design flaws [3][4]. Some of the main flaws with orthopaedic implants are associated with their longevity, material properties, and mismatch with patient size and shape requirements [5][6]. Stress shielding is one of the main design flaws of load-bearing prostheses. This phenomenon occurs because bone is a self-healing material that requires load application to remodel itself, but a material with a higher modulus of elasticity (E) absorbs all the stress generated, leading to bone reabsorption and subsequent loosening of the implant [7].
In the case of bone defects, they can be caused by tumour resection, infections, complex fractures, and non-unions [8]. The most common treatment for bone defects is surgical intervention, where an autograft (bone taken from the patient’s body) is used to fill bone defect spaces [9]. However, due to their restricted availability, allografts (bone tissue from a deceased donor) are frequently used to treat critical-size defects [9]. Bone grafting is a common surgical procedure; it has been estimated that 2.2 million grafting procedures are performed worldwide each year . However, late graft rupture has been reported to be as high as 60% 10 years after the grafting procedure [10]. Allograft transplantation has a success rate of approximately 70%. The low success rate of allografts is caused by the prevalence of infection, rejection by the host’s immune system, fatigue fractures, delayed union, non-union, and incomplete graft resorption [11][12]. In the case of autografts, the disadvantages are increased post-operative morbidity, lack of available tissue, chronic pain, infection, nerve injury, and weakened bone donor graft sites [12][13].
To solve these grafting problems, several scaffold traditional techniques have been used without much success: solvent-casting particulate-leaching, gas foaming, fibre meshes (fibre bonding), phase separation, melt moulding, emulsion freeze drying, solution casting, and freeze drying [14]. Some of the disadvantages of traditional scaffold fabrication techniques are their lack of control over porosity characteristics, such as pore size, pore distribution, and interconnectivity; the toxic by-products of scaffold degradation; and their lack of consistent mechanical properties [14]. Hence, traditional techniques for bone reconstruction including grafting and prostheses are not sufficiently effective, which represent a medical challenge that comes with several limitations and risks [9]. Moreover, no material yet exists with the ideal properties for bone tissue replacement [15][16][17]. To overcome these issues, tissue engineering has focused on additive manufacturing technologies to produce the next generation of bone implants and scaffolds.
2. Additive Manufacturing
Additive manufacturing (AM) technologies, supported by computer-aided design (CAD) software, progressively build 3D physical objects from a series of cross-sections, which are joined together to create a final shape [18]. With AM, it is possible to create complex interconnected and porous structures with controlled pore size, shape, and distribution and properties resembling bone mechanical properties, such as a modulus of elasticity to induce bone ingrowth [19][20]. This capability permits the fabrication of hierarchical structures at the microscale and the manipulation of material properties to create metamaterials. In terms of implant design, this advance means that products can be designed with a biomimetic approach according to the patient’s anatomy and the bone tissue’s mechanical properties [21]. The design freedom of AM allows its use in difficult clinical scenarios in which bone diseases, deformities, and trauma usually necessitate the reconstruction of bone defects with complex anatomical shapes, which is extremely difficult even for the most skilled surgeon [22]. The complex reconstruction of bone defects is possible through combining the advantages of AM with CAD and medical imaging technologies, such as computed tomography and magnetic resonance, to fabricate implants according to the patient’s specific anatomy, thus achieving an exact adaptation to the region of implantation [23]. In the search of suitable materials for AM, bone regeneration, and implant application tissue engineering has focused on developing a variety of different types of synthetic and natural materials.
3. Materials for Bone Regeneration and Implant Applications
Materials appropriate for implantation within the human body require distinct biocompatible properties. Therefore, in the selection of appropriate materials for implant applications, several factors must be considered. First, the intended implant location must be considered to predict host response, which is governed by the biochemical and physical environments in contact with the medical device [24][25]. Second, the material should possess appropriate biological and mechanical properties for its specific purpose to prevent physical damage to the body. Third, from the perspective of tissue engineering, materials should mimic one or multiple characteristics of the natural region of repair. In the case of bone repair, the desired characteristics are osteoconductivity, osteoinductivity, and osseointegration. As a result, for an optimum scaffold and prosthesis design, material science may combine several technologies to create suitable materials that fulfil these needs.
3.1. Polymers
Polymers for AM and tissue engineering applications are biocompatible materials that offer several advantages over other materials, including biodegradability, cytocompatibility, easy processability, and flexibility in the tailoring of their properties [26]. Polymers can be classified as natural or synthetic and some of them already have regulatory approval [27].
Natural polymers are made from proteins such as alginate, gelatine, collagen, silk, chitosan, cellulose, and hyaluronic acid [28]. The advantages of natural polymers are their excellent biodegradability, low production costs, and superior chemical versatility, as well as their improved biological performance that allow better interactions with cells than other biomaterials, improving their attachment and differentiation [29]. However, natural polymers can be expensive to produce, due to the difficulty in controlling their mechanical properties, biodegradation rate, and quality consistency[30].
Due to the disadvantages of natural polymers, different synthetic polymers, such as polycaprolactone (PCL), polylactic acid (PLA), and poly Lactic-co-Glycolic Acid (PLGA), have been developed. Their advantages include low immunogenic potential, large scale low production cost, and good quality consistency [31]. Moreover, their mechanical properties, microstructure, and degradation rate can be tuned according to needs [27]. Despite the advantages of natural and synthetic polymers, they are unsuitable for load-bearing applications due to their lower modulus of elasticity compared to bone, unstable mechanical strength, and tendency to creep [32][33]. Hence, in recent years, a variety of polymers have been combined with different materials to such as bioceramics (e.g., bioglasses, tri-calcium phosphates, and carbon nanotubes) and metals to create composite materials with tunable mechanical properties as well as with the capacity to deliver drugs, exosomes, and growth factors, to name a few [34][35][36][37].
3.2. Bioceramics
Bioceramics are a large group of materials used for bone substitution and regeneration. Calcium phosphate (CaP) ceramics is one of the main groups of bioceramics. Calcium phosphate ceramics are abundant in bone, constituting between 80% and 90% of bone’s anorganic matter. This group of bioceramics is widely used as implant coating, bone grafting, and more recently have been fabricated for bone scaffolding applications with AM [38][39]. Hydroxyapatite HAP and β-tricalcium phosphate (β-TCP), are the most-studied CaP bioceramics. The main advantages of calcium phosphate materials are their osteoinductive and osteoconductive properties, as well as their dissolution in body fluids [40]. For load-bearing applications, the major disadvantage of CaPs is their poor mechanical properties. Despite their good compressive strength, CaPs lack plastic deformation, making them brittle and prone to cracking. Consequently, these materials are not yet suitable for load-bearing applications [41]. Nevertheless, the lower wear rate of CaPs makes these materials the preferred choice for surface coating to reduce wear in joint prostheses [42]. They are also commonly used for spinal fusion, maxillofacial and cranio-maxillofacial reconstruction, as well as bone filler and bone cement due to their excellent biocompatibility and osteoconductivity [43].
Discovered in 1969 by Larry Hench, bioglasses are ceramic materials composed of calcium, phosphorus, and silicon dioxide [16]. Bioglasses are bioactive ceramic materials with strong osteointegrative and osteoconductive properties, as well as higher mechanical strength than calcium phosphate ceramics [44]. Hence, bioglasses have been intensely investigated with AM for bone tissue engineering applications [45][46]. The advantage of these materials is that by changing the proportions of their basic components, different forms with different properties can be obtained; for example, non-resorbable bioglasses can be transformed into resorbable bioglasses [44]. Moreover, they can be designed with controlled biodegradability and drug and cell delivery capabilities [47][48]. Their applications also include bioglass scaffolds produced using AM with controlled porosity architecture and improved mechanical properties for bone regeneration [49]. However, bioglasses are limited for use in practical load-bearing applications due to their low resistance to cyclic loading and their brittleness [50].
3.3. Metals and Titanium as a Bio-Metamaterial
Metals have been the common choice to replace hard tissue in load-bearing applications due to their mechanical properties, corrosion resistance, and biocompatibility. Most of these materials are alloys, such as 316L stainless steel (316LSS), cobalt chromium (Co–Cr), and titanium (Ti) alloys . Among all metallic materials, the titanium alloy Ti–6Al–4V is the gold standard for orthopaedic applications [51][52] because of its high biocompatibility [53], high corrosion resistance, low modulus of elasticity, and high strength-to-weight ratio. Furthermore, Ti is a reactive metal that naturally forms a thin layer of oxide, which blocks metal ions from reaching its surface, increasing its biocompatibility [54]. The biomedical applications of Ti–6Al–4V are quite broad and encompass dental implants; hip, shoulder, knee, spine, elbow, and wrist replacements; bone fixation components; and cardiovascular applications.
Nevertheless, the most common problems of metallic materials are wear and the stress-shielding effect caused by their high modulus of elasticity compared with bone [55][56][57]. Moreover, despite the excellent biocompatibility and mechanical properties of Ti and Ti alloys, they usually require long healing periods to create a stable interface with the surrounding bone [58], with insufficient implant osseointegration as a potential outcome [59]. Hence, to further augment the biological, mass transport, and mechanical performance of Ti and Ti alloys different metamaterials have been developed. For example, metallic bone implants with a modulus of elasticity similar to that of bone can drastically reduce wear, shear stress, and bone resorption and consequently prevent implant loosening and revision surgery [60]. This may translate into enhanced quality of life for the patient, reductions in hospital expenses and recovery time, and improvement in joint dynamic performance [61]. With porous Ti and Ti alloy bio-metamaterials, osseointegration is also improved, and superior results have been accomplished in relation to mechanical properties. Nonetheless, pores act as stress concentrators, reducing the material load capacity. As a result, for the design of load-bearing prostheses, it is crucial to balance mechanical properties with biological stimulation. Consequently, there have been several efforts to find the optimal balance between pore size and porosity percentage in different materials. For example, Zaharin et al. [62] investigated the effect of pore variation on the porosity and mechanical properties of several Ti–6A–l4V porous scaffolds. According to their results, scaffolds based on cube and gyroid unit cells with a pore size of 300 µm provided similar properties to bone. Moreover, they concluded that increments in porosity decreased the scaffolds’ elastic modulus and yield strength. In an earlier study Bobyn at al. [63] investigated the effects of pore size variation of cobalt-base alloy implants on the rate of bone growth. For this purpose, casted cobalt-base alloy implants were coated with powder particles and implanted into canine femurs for several weeks. The results indicated that pore sizes between 50 and 400 µm provided the maximum bone ingrowth and fixation strength.
Despite the excellent biocompatibility and mechanical properties of Ti and Ti alloys, they usually require long healing periods to create a stable interface with the surrounding bone, frequently resulting in insufficient osseointegration [64]. Hence, to further augment Ti’s bioactivity, corrosion resistance, and mechanical properties different mechanical, chemical, and physical surface modification methods have been developed [65][66][67]. Depending on the surface treatment used to modify Ti substrate, different topographic features can be achieved at the macroscale, microscale, and nanoscale. There is a large amount of evidence that rough Ti surfaces with topographic microfeatures better protein adsorption and provide higher osteoblasts attachment growth proliferation and activity than surface smooth surfaces [68]. Nonetheless, it has been demonstrated that nanoscale topography outperforms macro and micro-scale surface features towards augmenting cellular functions [69]. More recently, at has been proposed that a combination of different topographic features at the macro, micro, and nanoscale with local drug delivery functions can further enhance the biological, chemical, tribological, and mechanical performance of Ti bone implants [70][71][72][73].
4. Purpose and Objectives
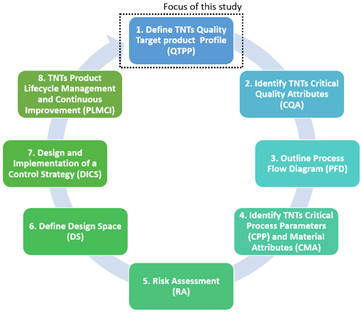
The purpose of this research is to provide researchers and industry with an in-depth adaptation of the Quality by Design (QbD) system for the fabrication of additively manufactured porous Ti implants considering the QbD guidelines for 3D printed bone implants and scaffolds . The QbD system is composed by eight main steps that need to be systematically followed to acquire a complete comprehension of the product and its manufacturing process, including the identification and control of all variables to achieve the desired quality. Specifically, the scope of this present study was limited to the first step of the QbD framework (Figure 1). Thus, the objectives of this study are:
- Define the ideal mechanical, geometrical and dimensional characteristics of the internal architecture of Ti bone scaffolds from a biomimetic perspective.
- Compare the results of different studies on fully porous Ti structures in relation to the ideal quality attributes of bone scaffolds.
- Identify the studies on fully porous Ti structures that satisfies the critical quality attributes of Ti porous bone implants and scaffolds.
Figure 1. Schematic showing the focus of this study within the QbD system.
This entry is adapted from the peer-reviewed paper 10.3390/ma13214794
References
- Cox, S.C.; Thornby, J.A.; Gibbons, G.J.; Williams, M.A.; Mallick, K.K. 3D printing of porous hydroxyapatite scaffolds intended for use in bone tissue engineering applications. Mater. Sci. Eng. C 2015, 47, 237–247, doi:10.1016/j.msec.2014.11.024.
- Reimann, P.; Brucker, M.; Arbab, D.; Lüring, C. Patient satisfaction - A comparison between patient-specific implants and conventional total knee arthroplasty. J. Orthop. 2019, 16, 273–277, doi:10.1016/j.jor.2019.03.020.
- Wafa, H.; Grimer, R.J. Surgical options and outcomes in bone sarcoma. Expert Rev. Anticancer Ther. 2006, 6, 239–248, doi:10.1586/14737140.6.2.239.
- FDA, U. Understanding Barriers to Medical Device Quality; US Food and Drug Administration: Silver Spring, MD, USA, 2011.
- Geetha, M.; Singh, A.; Asokamani, R.; Gogia, A. Ti based biomaterials, the ultimate choice for orthopaedic implants–a review. Prog. Mater. Sci. 2009, 54, 397–425, doi:10.1016/j.pmatsci.2008.06.004.
- Maji, P.K.; Banerjee, A.J.; Banerjee, P.S.; Karmakar, S. Additive manufacturing in prosthesis development–a case study. Rapid Prototyp. J. 2014, 20, 480–489, doi:10.1108/rpj-07-2012-0066.
- Katti, K.S. Biomaterials in total joint replacement. Colloids Surfaces B Biointerfaces 2004, 39, 133–142, doi:10.1016/j.colsurfb.2003.12.002.
- Calori, G.; Mazza, E.; Colombo, M.; Ripamonti, C. The use of bone-graft substitutes in large bone defects: any specific needs? Injury 2011, 42, S56–S63, doi:10.1016/j.injury.2011.06.011.
- Griffin, K.S.; Davis, K.M.; McKinley, T.O.; Anglen, J.O.; Chu, T.-M.G.; Boerckel, J.D.; Kacena, M.A. Evolution of bone grafting: Bone grafts and tissue engineering strategies for vascularized bone regeneration. C Clin. Rev. Bone Miner. Metab. 2015, 13, 232–244, doi:10.1007/s12018-015-9194-9.
- Cancedda, R.; Giannoni, P.; Mastrogiacomo, M. A tissue engineering approach to bone repair in large animal models and in clinical practice. Biomaterials 2007, 28, 4240–4250, doi:10.1016/j.biomaterials.2007.06.023.
- Hornicek, F.J.; Gebhardt, M.C.; Tomford, W.W.; Sorger, J.I.; Zavatta, M.; Menzner, J.P.; Mankin, H.J. Factors affecting nonunion of the allograft-host junction. Clin. Orthop. Relat. Res. 2001, 382, 87–98, doi:10.1097/00003086-200101000-00014.
- Burchardt, H. The biology of bone graft repair. Clin. Orthop. Relat. Res. 1983, 174, 28–34, doi:10.1097/00003086-198304000-00005.
- Seiler 3rd, J.; Johnson, J. Iliac crest autogenous bone grafting: donor site complications. J. South. Orthop. Assoc. 1999, 9, 91–97.
- Sachlos, E.; Czernuszka, J. Making tissue engineering scaffolds work. Review: the application of solid freeform fabrication technology to the production of tissue engineering scaffolds. Eur. Cells Mater. 2003, 5, 39–40, doi:10.22203/ecm.v005a03.
- Saijo, H.; Igawa, K.; Kanno, Y.; Mori, Y.; Kondo, K.; Shimizu, K.; Suzuki, S.; Chikazu, D.; Iino, M.; Anzai, M. Maxillofacial reconstruction using custom-made artificial bones fabricated by inkjet printing technology. J. Artif. Organs 2009, 12, 200–205, doi:10.1007/s10047-009-0462-7.
- Lichte, P.; Pape, H.; Pufe, T.; Kobbe, P.; Fischer, H. Scaffolds for bone healing: concepts, materials and evidence. Injury 2011, 42, 569–573, doi:10.1016/j.injury.2011.03.033.
- Parthasarathy, J. 3D modeling, custom implants and its future perspectives in craniofacial surgery. Ann. Maxillofac. Surg. 2014, 4, 9–18, doi:10.4103/2231-0746.133065.
- Prince, J.D. 3D Printing: An industrial revolution. J. Electron. Resour. Med Libr. 2014, 11, 39–45, doi:10.1080/15424065.2014.877247.
- Ryan, G.E.; Pandit, A.S.; Apatsidis, D.P. Porous titanium scaffolds fabricated using a rapid prototyping and powder metallurgy technique. Biomaterials 2008, 29, 3625–3635, doi:10.1016/j.biomaterials.2008.05.032.
- Barbas, A.; Bonnet, A.-S.; Lipinski, P.; Pesci, R.; Dubois, G. Development and mechanical characterization of porous titanium bone substitutes. J. Mech. Behav. Biomed. Mater. 2012, 9, 34–44, doi:10.1016/j.jmbbm.2012.01.008.
- Wong, K.-C.; Scheinemann, P. Additive manufactured metallic implants for orthopaedic applications. Sci. China Mater. 2018, 61, 440–454, doi:10.1007/s40843-017-9243-9.
- Martinez-Marquez, D.; Mirnajafizadeh, A.; Carty, C.P.; Stewart, R.A. Facilitating industry translation of custom 3d printed bone prostheses and scaffolds through Quality by Design. Procedia Manuf. 2019, 30, 284–291, doi:10.1016/j.promfg.2019.02.041.
- Martinez-Marquez, D.; Jokymaityte, M.; Mirnajafizadeh, A.; Carty, C.P.; Lloyd, D.; Stewart, R.A. Development of 18 quality control gates for additive manufacturing of error free patient-specific implants. Materials 2019, 12, 3110, doi:10.3390/ma12193110.
- Kulkarni, M.; Mazare, A.; Schmuki, P.; Iglič, A. Biomaterial surface modification of titanium and titanium alloys for medical applications. In Nanomedicine, Seifalian, A., de Mel, A., Kalaskar, D.M., Eds. One Central Press (OCP): Altrincham, UK, 2014; p. 111.
- Heness, G.; Ben-Nissan, B. Innovative bioceramics. Mater. Forum 2004, 27, 104–114.
- Singh, M.; Jonnalagadda, S. Advances in bioprinting using additive manufacturing. Eur. J. Pharm. Sci. 2020, 143, 105167, doi:10.1016/j.ejps.2019.105167.
- Donnaloja, F.; Jacchetti, E.; Soncini, M.; Raimondi, M.T.J.P. Natural and Synthetic Polymers for Bone Scaffolds Optimization. Polymers 2020, 12, 905, doi:10.3390/polym12040905.
- Asghari, F.; Samiei, M.; Adibkia, K.; Akbarzadeh, A.; Davaran, S. Biodegradable and biocompatible polymers for tissue engineering application: a review. Artif. Cells Nanomed. Biotechnol. 2017, 45, 185–192, doi:10.3109/21691401.2016.1146731.
- Rodríguez, G.R.; Patrício, T.; López, J.D. Natural polymers for bone repair. In Bone Repair Biomaterials; Elsevier BV: Amsterdam, The Netherlands, 2019; pp. 199–232.
- Haugen, H.J.; Lyngstadaas, S.P.; Rossi, F.; Perale, G. Bone grafts: which is the ideal biomaterial? J. Clin. Periodontol. 2019, 46, 92–102, doi:10.1111/jcpe.13058.
- Dwivedi, R.; Kumar, S.; Pandey, R.; Mahajan, A.; Nandana, D.; Katti, D.S.; Mehrotra, D. Polycaprolactone as biomaterial for bone scaffolds: Review of literature. JOBCR 2020, 10, 381–388, doi:10.1016/j.jobcr.2019.10.003.
- Liu, Y.; Lim, J.; Teoh, S.-H. Review: development of clinically relevant scaffolds for vascularised bone tissue engineering. Biotechnol. Adv. 2013, 31, 688–705, doi:10.1016/j.biotechadv.2012.10.003.
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554, doi:10.1016/j.tibtech.2012.07.005.
- Huang, B.; Vyas, C.; Roberts, I.; Poutrel, Q.-A.; Chiang, W.-H.; Blaker, J.J.; Huang, Z.; Bártolo, P. Fabrication and characterisation of 3D printed MWCNT composite porous scaffolds for bone regeneration. Mater. Sci. Eng. C 2019, 98, 266–278, doi:10.1016/j.msec.2018.12.100.
- De Moura, N.K.; Martins, E.F.; Oliveira, R.L.M.S.; de Brito Siqueira, I.A.W.; Machado, J.P.B.; Esposito, E.; Amaral, S.S.; de Vasconcellos, L.M.R.; Passador, F.R.; de Sousa Trichês, E. Synergistic effect of adding bioglass and carbon nanotubes on poly (lactic acid) porous membranes for guided bone regeneration. Mater. Sci. Eng. C 2020, 117, 111327, doi:10.1016/j.msec.2020.111327.
- De Witte, T.M.; Wagner, A.M.; Fratila‐Apachitei, L.E.; Zadpoor, A.A.; Peppas, N.A. Immobilization of nanocarriers within a porous chitosan scaffold for the sustained delivery of growth factors in bone tissue engineering applications. J. Biomed. Mater. Res. Part A 2020, 108, 1122–1135, doi:10.1002/jbm.a.36887.
- Swanson, W.B.; Zhang, Z.; Xiu, K.; Gong, T.; Eberle, M.; Wang, Z.; Ma, P.X. Scaffolds with Controlled Release of Pro-Mineralization Exosomes to Promote Craniofacial Bone Healing without Cell Transplantation. Acta Biomater. 2020, doi:10.1016/j.actbio.2020.09.052.
- Ghayor, C.; Weber, F.E. Osteoconductive Microarchitecture of Bone Substitutes for Bone Regeneration Revisited. Front. Physiol. 2018, 9, 960, doi:10.3389/fphys.2018.00960.
- Kim, J.-W.; Yang, B.-E.; Hong, S.-J.; Choi, H.-G.; Byeon, S.-J.; Lim, H.-K.; Chung, S.-M.; Lee, J.-H.; Byun, S.-H. Bone Regeneration Capability of 3D Printed Ceramic Scaffolds. Int. J. Mol. Sci. 2020, 21, 4837, doi:10.3390/ijms21144837.
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019, 23, 1–11, doi:10.1186/s40824-018-0149-3.
- Eliaz, N.; Metoki, N. Calcium Phosphate Bioceramics: A Review of Their History, Structure, Properties, Coating Technologies and Biomedical Applications. Materials 2017, 10, 334, doi:10.3390/ma10040334.
- Dorozhkin, S.V. Calcium orthophosphate deposits: Preparation, properties and biomedical applications. Mater. Sci. Eng. C 2015, 55, 272–326, doi:10.1016/j.msec.2015.05.033.
- Barrère, F.; van Blitterswijk, C.A.; de Groot, K. Bone regeneration: molecular and cellular interactions with calcium phosphate ceramics. I Int. J. Nanomed. 2006, 1, 317–332.
- Fernandes, H.R.; Gaddam, A.; Rebelo, A.; Brazete, D.; Stan, G.E.; Ferreira, J.M.J.M. Bioactive glasses and glass-ceramics for healthcare applications in bone regeneration and tissue engineering. Materials 2018, 11, 2530, doi:10.3390/ma11122530.
- Seidenstuecker, M.; Kerr, L.; Bernstein, A.; Mayr, H.O.; Suedkamp, N.P.; Gadow, R.; Krieg, P.; Hernandez Latorre, S.; Thomann, R.; Syrowatka, F.J.M. 3D powder printed bioglass and β-tricalcium phosphate bone scaffolds. Materials 2017, 11, 13, doi:10.3390/ma11010013.
- Ma, Y.; Dai, H.; Huang, X.; Long, Y. 3D printing of bioglass-reinforced β-TCP porous bioceramic scaffolds. J. Mater. Sci. 2019, 54, 10437-10446, doi:10.1007/s10853-019-03632-3.
- Mancuso, E.; Bretcanu, O.A.; Marshall, M.; Birch, M.A.; McCaskie, A.W.; Dalgarno, K.W. Novel bioglasses for bone tissue repair and regeneration: Effect of glass design on sintering ability, ion release and biocompatibility. Mater. Des. 2017, 129, 239-248, doi:10.1016/j.matdes.2017.05.037.
- Negut, I.; Floroian, L.; Ristoscu, C.; Mihailescu, C.N.; Mirza Rosca, J.C.; Tozar, T.; Badea, M.; Grumezescu, V.; Hapenciuc, C.; Mihailescu, I.N. Functional bioglass—biopolymer double nanostructure for natural antimicrobial drug extracts delivery. Nanomaterials 2020, 10, 385, doi:10.3390/nano10020385.
- Yang, J. Progress of bioceramic and bioglass bone scaffolds for load-bearing applications. I In Orthopedic Biomaterials; Springer: Berlin/Heidelberg, Germany, 2018; pp. 453–486.
- Rizwan, M.; Hamdi, M.; Basirun, W.J.J.o.b.m.r.P.A. Bioglass® 45S5‐based composites for bone tissue engineering and functional applications. J. Biomed. Mater. Res. Part A 2017, 105, 3197–3223, doi:10.1002/jbm.a.36156.
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R: Rep. 2004, 47, 49–121, doi:10.1016/j.mser.2004.11.001.
- Rack, H.; Qazi, J. Titanium alloys for biomedical applications. Mater. Sci. Eng. C 2006, 26, 1269–1277, doi:10.1016/j.msec.2005.08.032.
- Niinomi, M.; Nakai, M.; Hieda, J. Development of new metallic alloys for biomedical applications. Acta Biomater. 2012, 8, 3888–3903, doi:10.1016/j.actbio.2012.06.037.
- Vandrovcová, M.; Bacakova, L. Adhesion, growth and differentiation of osteoblasts on surface-modified materials developed for bone implants. Physiol. Res. 2011, 60, 403–417, doi:10.33549/physiolres.932045.
- Huiskes, R.; Weinans, H.; Van Rietbergen, B. The Relationship Between Stress Shielding and Bone Resorption Around Total Hip Stems and the Effects of Flexible Materials. Clin. Orthop. Relat. Res. 1992, 274, 124–134, doi:10.1097/00003086-199201000-00014.
- Ryan, G.; Pandit, A.; Apatsidis, D.P. Fabrication methods of porous metals for use in orthopaedic applications. Biomaterials 2006, 27, 2651–2670, doi:10.1016/j.biomaterials.2005.12.002.
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R Rep. 2015, 87, 1–57, doi:10.1016/j.mser.2014.10.001.
- Strnad, J.; Strnad, Z.; Šesták, J.; Urban, K.; Povýšil, C. Bio-activated titanium surface utilizable for mimetic bone implantation in dentistry—Part III: Surface characteristics and bone–implant contact formation. J. Phys. Chem. Solids 2007, 68, 841–845, doi:10.1016/j.jpcs.2007.02.040.
- Zhao, X.; Wang, T.; Qian, S.; Liu, X.; Sun, J.; Li, B. Silicon-doped titanium dioxide nanotubes promoted bone formation on titanium implants. Int. J. Mol. Sci. 2016, 17, 292, doi:10.3390/ijms17030292.
- Wiria, F.E.; Maleksaeedi, S.; He, Z. Manufacturing and characterization of porous titanium components. Prog. Cryst. Growth Charact. Mater. 2014, 60, 94–98, doi:10.1016/j.pcrysgrow.2014.09.001.
- Zhang, L.C.; Klemm, D.; Eckert, J.; Hao, Y.L.; Sercombe, T.B. Manufacture by selective laser melting and mechanical behavior of a biomedical Ti–24Nb–4Zr–8Sn alloy. Scr. Mater. 2011, 65, 21–24, doi:10.1016/j.scriptamat.2011.03.024.
- Zaharin, H.A.; Rani, A.; Majdi, A.; Azam, F.I.; Ginta, T.L.; Sallih, N.; Ahmad, A.; Yunus, N.A.; Zulkifli, T.Z.A. Effect of unit cell type and pore size on porosity and mechanical behavior of additively manufactured Ti6Al4V scaffolds. Materials 2018, 11, 2402, doi:10.3390/ma11122402.
- Bobyn, J.; Pilliar, R.; Cameron, H.; Weatherly, G.J.C.O.; Research®, R. The optimum pore size for the fixation of porous-surfaced metal implants by the ingrowth of bone. Clin. Orthop. Relat. Res. 1980, 150, 263–270, doi:10.1097/00003086-198007000-00045.
- John, A.A.; Jaganathan, S.K.; Supriyanto, E.; Manikandan, A. Surface modification of titanium and its alloys for the enhancement of osseointegration in orthopaedics. Curr. Sci. 2016, 111, 1003-1015, doi:10.18520/cs/v111/i6/1003-1015.
- Zhang, L.-C.; Chen, L.-Y.; Wang, L. Surface Modification of Titanium and Titanium Alloys: Technologies, Developments, and Future Interests. Adv. Eng. Mater. 2020, 22, 1901258, doi:10.1002/adem.201901258.
- Sasikumar, Y.; Indira, K.; Rajendran, N. Surface Modification Methods for Titanium and its alloys and their corrosion behavior in biological environment: a review. J. Bio. Tribo-Corros. 2019, 5, 36, doi:10.1007/s40735-019-0229-5.
- Civantos, A.; Martinez-Campos, E.; Ramos, V.; Elvira, C.; Gallardo, A.; Abarrategi, A. Titanium coatings and surface modifications: toward clinically useful bioactive implants. ACS Biomater. Sci. Eng. 2017, 3, 1245–1261, doi:10.1021/acsbiomaterials.6b00604.
- Zhang, K.; Fan, Y.; Dunne, N.; Li, X. Effect of microporosity on scaffolds for bone tissue engineering. Regen. Biomater. 2018, 5, 115–124, doi:10.1093/rb/rby001.
- Staruch, R.; Griffin, M.F.; Butler, P. Nanoscale Surface Modifications of Orthopaedic Implants: State of the Art and Perspectives. Open Orthop. J. 2016, 10, 920–938, doi:10.2174/1874325001610010920.
- Zemtsova, E.; Arbenin, A.; Valiev, R.; Smirnov, V. Modern techniques of surface geometry modification for the implants based on titanium and its alloys used for improvement of the biomedical characteristics. In Titanium in Medical and Dental Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 115–145.
- Gulati, K.; Prideaux, M.; Kogawa, M.; Lima‐Marques, L.; Atkins, G.J.; Findlay, D.M.; Losic, D. Anodized 3D–printed titanium implants with dual micro‐ and nano‐scale topography promote interaction with human osteoblasts and osteocyte‐like cells. J. Tissue Eng. Regen. Med. 2016, 11, 3313–3325, doi:10.1002/term.2239.
- Zafar, M.S.; Fareed, M.A.; Riaz, S.; Latif, M.; Habib, S.R.; Khurshid, Z. Customized Therapeutic Surface Coatings for Dental Implants. Coatings 2020, 10, 568, doi:10.3390/coatings10060568.
- Kunrath, M.F.; Leal, B.F.; Hubler, R.; de Oliveira, S.D.; Teixeira, E.R. Antibacterial potential associated with drug-delivery built TiO2 nanotubes in biomedical implants. AMB Express 2019, 9, 1-13, doi:10.1186/s13568-019-0777-6.