Several studies showed that the gut–brain axis influences the development of anxiety and depression. The gut–brain axis is a network that transmits information in a bidirectional pattern between the gut and the brain and is controlled by neuroendocrine and neuroimmune mechanisms [
63,
64,
65]. Gamma-aminobutyric acid (GABA) neurotransmitters [
66], secondary bile acids [
67], short-chain fatty acids [
68], and tryptophan metabolites generated from the microbiota are only a few of the molecules that regulate these mechanisms [
66,
69,
70]. The gut–brain axis is dysregulated and linked to neuroinflammation and altered blood–brain barrier (BBB) permeability during gut microbiota dysbiosis or disturbance in the gut ecosystem [
71]. According to research using rodent models, the BBB becomes more permeable when the normal intestinal microbiota is lost or disturbed [
72]. Increased BBB permeability and possible subsequent development of Alzheimer’s disease with amyloid-peptide accumulation may be associated with metabolic illnesses [
73]. It has been discovered that microbial dysbiosis affects the protective properties of the BBB, including permeability modulation [
72] via tight junction expression [
74], and causes behavioral alterations [
75].
Studies showed that changes in gut microbiota increased the level of harmful compounds such as
p-cresol, which may compromise the integrity of the BBB [
76,
77]. Earlier research demonstrated that
p-cresol was considerably higher in the prefrontal cortex of susceptible mice that previously exhibited anxiety-like phenotypes [
78]. Moreover, the gut-derived metabolite 4-ethyl phenyl sulfate (4EPS) affects brain activity and causes anxiety-like behaviors [
79]. The gut microbiota generates reduced levels of neurotoxic metabolites after administration of
Bacteroides fragilis, including 4-EPS, serum glycolate, and imidazole propionate, improving gut permeability and reducing anxiety-like behavior [
80]. Serotonin and dopamine release, brain-derived neurotrophic factor levels, the HPA axis, and the production of inflammatory cytokines may all be affected by disturbances in the gut microbiota during depression and anxiety [
81]. For instance, C-reactive protein (CRP) and cytokines, including interleukin-1, interleukin-2, interleukin 6, interleukin-1β, and interferon-γ, were released in response to depression [
82].
Microorganisms inhabit the human gut in a synbiotic manner. The prokaryotic organisms that comprise the “human holobiont” are essential for preserving homeostasis and proper functioning. Various neurological illnesses, including Alzheimer’s disease, Parkinson’s disease, depression, etc., have been related to disturbances in the gut microbiota composition. Numerous sophisticated molecular mechanisms, including immune system modification [
91], metabolic signaling [
92], neuroendocrine signaling [
93], vagal nerve signaling [
94], and epigenetics, are used by these microorganisms to maintain normal homeostasis [
95,
96]. Epigenetics plays a significant role in controlling host physiology by modifying the metabolic activity of the gut microbiome, which is influenced by environment and nutrition. For example, cofactors for the activity of enzyme acetylases and methylases, which control histone modification and DNA methylation, originate from the gut microbiome. The metabolites generated by the gut microbiota function as cofactors and substrates for numerous enzyme activities [
95]. Epigenetic regulation is a dynamic process affected by changes in diet, activity, and microbiota composition [
97]. Epigenetics means “in addition to genetics”. Instead of looking at the DNA sequence, it includes analyzing chromosome-level changes in gene expression. Both modifications are persistent and heritable. Epigenetics primarily control chromosomal superstructure changes and chemical modifications to nitrogenous bases without directly affecting the DNA sequence. Epigenetics may result from several molecular processes, but the primary ones include histone modification, DNA methylation and acetylation, and RNA-associated silencing [
98].
2. Potential Therapy Involved in the Treatment of Depression and Anxiety
Human depression may be treated with various synthesized drugs, though their effectiveness varies depending on several factors [
180]. The recent alternative potential strategies currently being widely considered include modified diets, fish and omega-3 fatty acids intake, probiotics, prebiotics, synbiotics, postbiotics, fecal microbiota transplantation, and 5-hydroxytryptophan regulation (
Table 2). These approaches directly or indirectly restore healthy gut microbial composition and diversity.
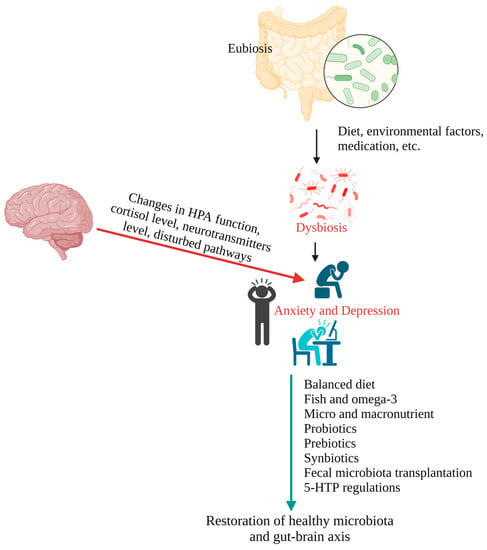
Figure 2 represents the different methods of restoration of gut microbiota to prevent and treat anxiety and depression.
Figure 2. Illustration representing the factors associated with the development of anxiety and depression and the methods of gut microbiota restoration for the prevention and treatment of anxiety and depression.
Table 2. Supplements that aid in the management of depression and anxiety.
2.1. Modified Diet
Nutritional study has shifted away from concentrating on individual nutrients since they are hardly taken in the isolated form [
197]. According to research, nutrient-dense food supports physical and mental health [
138]. Proper brain function is supported by dietary nutrients such as vitamins, minerals, polyunsaturated fats, and amino acids in a balanced diet [
140,
141]. Numerous nutrients act as enzyme cofactors, producing neurotransmitters, cell signaling, and metabolic pathways [
198]. Several different diets might aid in reducing anxiety and sadness. A decreased risk of anxiety or depression was linked to consuming a Mediterranean diet [
199,
200,
201]. In individuals with low levels of anxiety and depression, this diet exhibits a preventive effect against unfavorable cardiovascular disease events [
201]. The Mediterranean diet assures enough essential nutrients to prevent depression, such as fruits, nuts, vegetables, grains, legumes, and seafood. Intake of folate was negatively correlated with the frequency of depression in males, particularly smokers. Intake of the B12 vitamin was negatively correlated with depression in women, particularly in smokers and physically active women [
202]. In addition, the Mediterranean diet alters the gut flora [
203], which may be a plausible mechanism for reducing anxiety and depression. With an exposure–response connection, the “healthy Japanese” pattern may be inversely related to depressive symptoms. In addition to vegetables, fish/shellfish, and fruit, the “healthy Japanese” diet also featured potatoes, seaweed, mushrooms, and soy products. These seem to provide a dietary pattern less likely to cause inflammation. This aspect may be linked to improved psychological health via the production of monoamines and gut flora [
204]. Even a fiber-rich diet reduces intestinal pH, preventing harmful bacteria from overgrowing [
205]. Extensive research has shown the potential advantages of prebiotics, probiotics, and special dietary therapies in treating depression by modulating gut microbiota and depression through the gut–brain axis [
206,
207].
2.2. Fish and Omega-3 FA Intake
Healthy dietary habits that include fish have been linked to a decreased incidence of depression [
22,
208,
209,
210]. Both clinical and preclinical investigations have shown that fish oil is rich in omega-3 and exhibits antidepressant properties [
211,
212]. A meta-analysis of 13 randomized clinical trials found that fish oil demonstrated potential for treating serious depression [
213]. A supplemental diet rich in omega-3 and omega-6 polyunsaturated fatty acids boosts
Bifidobacterium and
Lactobacillus and controls microbial metabolism, particularly during early life stress [
212,
214]. Omega-3 fatty acids, such as docosahexaenoic acid and eicosapentaenoic acid, have also been shown to improve cognition in adulthood and reduce stress and depression [
212,
215].
2.3. Micronutrient Intake
Many micronutrients are provided through the host’s diet and are also needed for the microbes. Therefore, the host’s micronutrient consumption may impact the composition and functioning of the intestinal flora. Mice receiving a diet low in magnesium had altered gut flora, which was linked to increased depression-like phenotypes [
216]. Many bacteria need iron; therefore, iron consumption via diet impacts the diversity of the intestinal flora [
217]. In addition, iron deficiency also has an impact on some neurotransmitter levels in the hippocampus and the corpus striatum [
218]. People with depression are more iron-deficient than healthy individuals [
219]. This result may explain the need for iron to produce neurotransmitters implicated in the pathophysiology of depression. Vitamin B alleviates anxiety, depression, and stress [
173,
174,
220], and vitamin D3 alleviates depression [
221,
222,
223,
224].
2.4. Macronutrient Intake
A greater incidence of depression is substantially linked to a lower protein consumption than recommended. A 10% increase in protein consumption was shown to reduce the incidence of depression considerably in South Korea and in the United States [
225]. Several biological explanations have linked the intake of protein and depression. These theories are supported by the fact that tryptophan, an amino acid, is a precursor of serotonin. Although consuming a large amount of protein may raise the plasma content of tryptophan [
226], other neural amino acids can compete with tryptophan for brain cellular absorption [
227]. As a result, increased protein consumption does not always result in higher levels of tryptophan in the brain. The finding that increased protein consumption protects against depression through boosting serotonin in the brain is challenging to understand because of this contradictory impact of protein intake on the tryptophan content. Additionally, other macronutrients have the power to control tryptophan levels and synthesis; for example, consuming carbs or receiving an insulin injection has been shown to raise plasma tryptophan levels [
228]. Focusing on the impacts of macronutrients on the intestinal flora has led to an increasing convergence in nutrition [
229]. Plant protein, unsaturated fats, and fiber encourage a healthy gut flora compared to excessive animal protein intake, saturated fats, and simple or artificial carbohydrates.
The quality of macronutrients, particularly dietary carbohydrates, is another factor to consider. The impact of high- and low-glycemic-load meals on depression symptoms was investigated in nondepressed persons. According to this research, eating a diet high in glycemic load may result in overall mood changes, more tiredness, and depression symptoms than eating a diet low in glycemic load [
230]. The physiological effects of fatty acids vary depending on their type. However, no clinical trial data are available on how fatty acids affect depression or depressed symptoms depending on their saturation level. Since inflammation and endothelial dysfunction are significant risk factors for depression and cardiovascular disease, dietary advice for preventing cardiovascular disease may be beneficial for managing and preventing depression. The prevention and treatment of depression may be aided by replacing saturated fats with unsaturated fatty acids [
231], although more research is required to confirm this statement.
2.5. Probiotics
Probiotics, living microorganisms, encourage the development of beneficial bacteria [
54,
232,
233]. When the probiotic
L. rhamnosus was administered to stressed mice, it decreased corticosterone levels and the stress-induced gamma-aminobutyric acid 2 mRNA expression. It did not affect gamma-aminobutyric acid 2 expression in the hippocampus [
92,
234]. Treatment with the probiotic
L. farciminis reduced gut barrier leakiness [
91]. In animal models, administering a probiotic such as
Bifidobacterium longum restored hippocampus BDNF levels and decreased inflammation-induced anxiety-like behavior [
183,
234]. Probiotics have been observed to lessen depressive-like behavior in IBS patients, but not anxiety [
235]. The
Oscillibacter strain aids in treating insomnia and anxiety [
236,
237].
B. longum is beneficial in reducing stress-induced cortisol levels and daily self-reported stress levels [
238]. Probiotics including
B. bifidum,
B. lactis,
Lactococcus lactis,
L. casei,
L. salivarius,
L. brevis, and
L. acidophilus showed promising results in reducing negative thoughts and behavior in another study [
239]. These results from studies demonstrate that probiotics may be used to treat depression and anxiety.
2.6. Prebiotics
Prebiotics are specific substrates that support the development and activity of certain advantageous gut microorganisms [
240,
241]. In healthy young volunteers, dietary prebiotics, including fructo-oligosaccharides (FOS) and galacto-oligosaccharides (GOS), encouraged the growth of advantageous bacteria such as
B. longum. They decreased the hypothalamic–pituitary–adrenal axis activation caused by stress [
206,
242,
243]. Rats’ anxiety and depressive-like behavior caused by lipopolysaccharides were decreased after receiving GOS [
207]. Crocin-I enhanced gut microbiota composition and short-chain fatty acid levels and improved the brain-derived neurotrophic factor level in mice with depression-like phenotypes [
244].
2.7. Synbiotics
Synbiotics may improve gut microbial activity. Malondialdehyde and hydrogen peroxide concentrations in human plasma were significantly reduced after taking syn-biotics [
245]. Women consuming synbiotics had considerably greater plasma levels of glutathione and free sulfhydryl groups than males [
246,
247]. According to a randomized trial, synbiotic FOS, GOS, and inulin combined with a probiotic mixture containing
B. lactis,
B. bifidum,
L. acidophilus, and
B. longum reduced depression and increased serum levels of a brain-derived neurotrophic factor in depressed patients compared to controls. According to this study, synbiotics reduced depressive symptoms more than probiotics alone [
181].
2.8. Postbiotics
Postbiotics have therapeutic effects similar to those of probiotics in that they support the integrity of the epithelial barrier function, restore the microbiota’s diversity and composition, control immunological responses, and regulate signaling along the gut–brain axis [
248,
249]. The administration of a heat-killed
L. helveticus strain reduced anxiety- or depression-like phenotypes in adolescent male mice. It improved the genes involved in neuron differentiation and development and signal transduction in the nucleus accumbens [
250]. Adult male mice were given heat-killed
Enterococcus fecalis along with diet, which decreased depressive and anxious-like behaviors, increased the expression of the genes for the neurotransmitter receptors, and increased the density of
Butyricicoccus and
Enterococcus content in the gut [
251]. Young individuals subjected to chronic stress were given two tablets of heat-inactivated
L. gasseri daily for 24 weeks to lower their anxiety, improve their sleep, produce more n-valeric acid, and restore the balance of their microbiome [
252].
2.9. Fecal Microbiota Transplantation (FMT)
FMT repairs gut diversity by transferring healthy microflora to the patient’s gut. FMT was developed to achieve healthy gut microbial composition and function, much like probiotics. When healthy donors’ fecal microbiota was transferred to anxious mice, it resulted in a reduction in the symptoms of anxiety and depression. FMT is now one of the approaches most often used to treat gastrointestinal and neuropsychiatric diseases [
253]. IBS and other GI tract-related issues have been linked to depression in clinical trials. FMT from a healthy donor reduced IBS patients’ depressive and anxious-like behavior, and
Clostridium difficile infection was also reduced in older patients [
254,
255]. However, if fecal microbiota is transferred from an unhealthy individual, it may cause adverse side effects such as depression. For example, according to a report, fecal microbiota transferred from rheumatoid arthritis patients with depression caused depression-like behaviors in mice via systemic inflammation [
256]. Therefore, extra precautions are required for FMT procedures.
2.10. Bifidobacterium and 5-HTP Regulation
In a study, oral 5-HTP treatment markedly improved gut microbiota dysbiosis in mice exhibiting depression-like phenotypes. When 5-HTP was used to treat depression in rats, it helped maintain levels of short-chain fatty acids and brain-derived neurotrophic factors [
257]. In a different study, mice underwent a 5-week trial of chronic moderate stress and received LAB (
B. longum subsp.
infantis E41 and
B. breve M2CF22M7). E41 and M2CF22M7 dramatically decreased depression-like phenotypes in the mice by increasing Tph1 expression and 5-HTP secretion in RIN14B cells. 5-HTP and brain-derived neurotrophic factor concentrations in the brain were elevated after E41 and M2CF22M7 administration [
258].