Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biochemistry & Molecular Biology
The prolific applicability of nanomaterials has made them a common citizen in biological systems, where they interact with proteins forming a biological corona complex.
- protein corona
- ICP-MS
- nanomaterials
1. The Protein Corona Formation
Nanotechnology has experienced enormous growth in recent decades, bringing forward the surging use of engineered nanoparticles as advantageous novel materials in numerous fields such as biomedicine, cosmetics, pharmacology, food, or agriculture [1,2]. Its unrestrained used has resulted in the ubiquity of these nanomaterials in the environment [3] and biological systems [4], and thus nanoparticles may potentially be inhaled, ingested, or taken up through the skin into the body [5]. Then, when nanoparticles come in contact with biological fluids, they become covered by a complex layer of biomolecules, such as proteins, lipids, or sugars, forming a sort of “bio-corona” [6,7]. Within the biological corona complexes formed in biological systems, the predominant and most studied molecules are proteins, which on their own form the so-called nanoparticle protein corona complex (NPPC) [5,8]. The protein corona defines the biological identity of the nanoparticle, the NPPC being the entity that finally interacts with and is “seen” or recognized by the cell [6].
Upon introduction in a biological fluid, the process of protein adsorption is an almost instantaneous event given the higher binding energy of the nanoparticle surface compared to the surrounding biological environment (Gibbs free energy drives the complex stability) [9,10]. The formation of the corona, driven by non-covalent forces, is dynamic and changes over time because of continuous association and dissociation processes given the different affinity of the proteins. Initially, higher concentration proteins will interact with the nanoparticle, but will eventually be displaced by higher-affinity proteins until reaching an equilibrium, when protein exchanges would not affect the composition of the corona [6,9,11,12]. In fact, although the formation of the corona takes place over the period of an hour, occurring minor changes for around 12 h [13], plasma proteins have been found in the corona already within the first minute of exposure [14]. Interestingly, the composition of the corona at such early stages does not significantly change over time in protein identities, but in quantities [14]. The dynamic nature of these phenomena can be described by using the “hard” and “soft” corona concepts. Those proteins with high affinity that form the closest layer to the nanoparticle surface are called the hard corona. This constitutes a tight, strong, nanoparticle–protein binding that is highly stable of very rapid formation. Low-affinity proteins forming an external layer, which are not bound to the nanoparticle surface but have a certain degree of interactions, are called the soft corona [4,6,9] (Figure 1). It takes more time to constitute, and it is more unstable and highly dynamic, being more complex in its study.
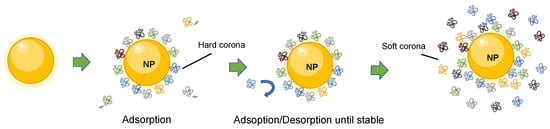
Figure 1. Scheme representation of the dynamic formation of nanoparticle–protein corona complex. Proteins in the inner layer have high affinity for the nanoparticle surface and form the hard corona. The proteins in the outer layer form the soft corona, which shows dynamic changes due to exchanges with free proteins in the environment.
Nanomaterial interactions with proteins can be controlled by different strategies, nanoparticle surface functionalization being the most common one [15]. This is because protein corona formation is a process commonly considered problematic, and thus great effort is being invested by the scientific community on designing and engineering coatings that minimize protein adsorption on nanomaterial surfaces as much as possible [16].
2. The Biological Impact of the Protein Corona
The interactions between nanoparticles and proteins have significant biological consequences to the original nanoparticle and to the native proteic environment. On the one hand, the formation of the protein corona on the nanoparticle surface changes the nanoparticle physicochemical properties. It changes the size, shape, and even the aggregation state of the nanoparticle, which in turn affects how proteins are oriented and presented to the biological targets [17]. On the other hand, interactions with nanoparticles may trigger protein aggregation or conformational changes, which may impact the protein functions and their interaction with other biomolecules, and may even result in an immune response to eliminate the circulating nanoparticles. In this regard, changes in protein stability and enzyme activity have been observed when immobilized on the nanoparticle surface [6].
Upon entrance in the biological media, nanoparticles are involved in a myriad of biological processes. The formation of the protein corona creates a different structure that will drive and influence the behavior and interaction of the nanoparticles with biological systems. Thus, the protein corona can alter the interactions of the nanoparticle with the cell surface moieties (e.g., antibodies), promoting or inhibiting the uptake of the nanoparticle by the cell [18]. For instance, in a case of study with silica nanoparticle, it was observed that the formation of the protein corona reduced the cell uptake by weakening their cell membrane adhesion [19]. The protein corona also plays an important role in targeting the nanoparticles to different organs [13].
The biological impact of the nanoparticle interactions with proteins is particularly relevant in the development and use of the NPPC in biomedicine. The small size of nanoparticles gives them the ability to efficiently access cell compartments, which has important biomedical implications, leading to important developments in the field of nanomedicine [20,21]. The feature of the protein corona, which can direct the nanoparticles to specific organs or locations, can be used to design NPPC that can be placed anywhere desired in the organism or in the cell. This final location will then determine the effect of the NPPC and the pathways or processes it gets involved in, disrupts, or promotes. Likewise, it can be used as a vehicle for drug delivery [18], or for the promoted accumulation of nanoparticles in specific areas, e.g., tumors, and serve as a biomarker or therapeutical target [9]. Naturally, the presence of nanoparticles in biological systems and their use in biomedicine brings a potential safety and toxicological risk. Even for nanoparticles without the protein corona, cellular uptake is related to cytotoxicity, being affected by properties like colloidal stability or nanoparticle surface charge. In fact, positively charged nanoparticles tend to have higher cellular uptake as well as a higher cytotoxicity than negatively charged nanoparticles [22].
Nowadays, it is unquestionable that the use of NPs in biological applications requires a precise knowledge of the NP–protein corona system (NPPC) that is formed when NPs enters biosystems. Consequently, NPs acquire a new biological identity through the formation of this protein corona, which affects their colloidal stability, biodistribution, interactions, toxicity, and clearance. The understanding of the fate of the NPPC in biological systems, the development of NPPCs with biomedical applications, or the understanding of the NPPC toxicological effect require accurate and reliable characterization of nanoparticle physicochemical properties, the protein corona composition, and the dynamics of the interaction between nanoparticles and the different proteins that constitute the corona. To this end, the combination of multiple analytical methodologies offering complementary information certainly seems to be necessary. Knowledge of nanoparticle protein corona composition commonly relies on approaches that lack standardization methods that guarantee or control the reproducibility and reliability of the results. In particular, important difficulties persist in the effective quantification of the different proteins from the corona. ICP-MS turns up as a complementary tool that could provide accuracy and robustness of quantitative results and workflows given its species-independent nature. In fact, thanks to the presence of ICP-detectable elements in widespread inorganic NPs (Au, Ag, Ti, Ce, Cd, Se, Zn, among other) and proteins (S), ICP-MS can provide valuable information, including NP elemental composition, size, concentration, and populations on the one hand, and absolute protein amounts on the other.
This entry is adapted from the peer-reviewed paper 10.3390/nano13061132
This entry is offline, you can click here to edit this entry!
