Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
(S100 calcium-binding protein B) S100B protein has emerged as the most widely studied and used biomarker for clinical decision making in patients with mTBI. In addition to its use as a diagnostic biomarker, S100B plays an active role in the molecular pathogenic processes accompanying acute brain injury.
- S100B
- biomarker
- actor
- traumatic brain injury
1. What Is the S100B Protein?
1.1. General Characteristics
S100B belongs to the S100 family, a family of small calcium-binding cytosolic proteins first described by Moore in 1965 [1]. The name is derived from the protein’s complete solubility in a saturated ammonium sulfate solution [2]. The S100 family consists of more than twenty members characterized by two calcium-binding sites with a helix–loop–helix (“EF-hand”) structure [3]. Calcium binding induces a conformational S100 change that exposes a hydrophobic surface, allowing recruitment of other proteins leading to a biological response [4]. S100B is a small dimeric protein (molecular weight 21 kDa) that consists of ββ or αβ chains [5], predominantly expressed by astrocytes, but also to some extent by other cells in the central nervous system (CNS), including oligodendrocytes, neural progenitor cells and certain neuronal populations [6][7][8]. Physiologically, the protein has both intracellular and extracellular functions, including the regulation of protein phosphorylation and enzyme activity, calcium homeostasis and the regulation of cytoskeletal components and transcriptional factors [3]. As an extracellular factor, S100B interacts with surrounding cell types through the receptor for advanced glycation end-products (RAGE) [9].
1.2. Release and Elimination
Under physiological conditions, S100B mainly produced by astrocytes do not cross the blood–brain barrier (BBB), and the concentration of S100B in the cerebrospinal fluid is reported to be about 100-fold higher than in serum [10]. After brain insults, S100B released from damaged glial cells can diffuse into the bloodstream [11]. The mechanisms underlying this diffusion across the BBB are not completely clear. Some authors claim that S100B is released in the serum through the disrupted BBB [12][13][14]. However, in studies focused on TBI patients, there was no correlation between BBB disruption and the peak levels of S100B [7][15]. Furthermore, it has been shown that the recently described glymphatic system may play an important role in the outflow of S100B from the brain [16].
After diffusion into the blood stream, S100B is subject to renal elimination [7]. Some authors have reported a modest S100B elevation in patients with renal failure [17][18][19]. However, mild-to-moderate renal failure has not been shown to significantly affect S100B levels in serum [20]. S100B is eliminated with a biological half-life of approximately 30 min [20]. Diseases such as malignant melanoma or TBI may affect the half-life, with values up to 90 min [21] and 97 min [22], respectively. Note that the S100B gene is located on chromosome 21, explaining an increase in blood concentration for patients affected by Down syndrome.
1.3. Extracranial Sources of S100B
S100B protein is not brain specific and extracranial contributions may influence the interpretation of the results in a clinical context. In effect, S100B is also expressed in melanocytes, chondrocytes, adipocytes and skeletal muscle [23][24][25]. It is also known that serum S100B levels are influenced by skin pigmentation [26], which can be explained by a moderate production in melanocytes under physiological conditions [27]. In a cohort of 136 healthy individuals divided into three groups according to ethnicity, Black and Asian individuals had higher serum S100B concentrations than Caucasians, with mean values of 0.14, 0.11 and 0.07 µg/L, respectively [28]. Increased serum S100B levels are also observed in patients with extracranial trauma, especially in patients with bone fractures, soft tissue trauma or thoracic injury [29][30]. These damages might confound the interpretation of elevated serum S100B levels as the protein is mainly released from peripheral sources such as adipocytes, chondrocytes and skeletal muscle cells [29]. The source of S100B protein elevations is probably multifactorial and, as a recent study suggests, is associated with overall trauma severity [30]. In the context of cardiac surgery, it has been shown that increases in serum S100B levels post-surgery are not only related to cerebral hypoperfusion but also to surgical wounds, probably from surgically traumatized fat, muscle and bone marrow [31]. Moreover, numerous studies have described an increase in serum S100B levels after sports activity [32]. In fact, physical exercise and associated hypoxia are reported to induce the cerebral synthesis and release of S100B [32][33][34]. The increase in blood–brain barrier (BBB) permeability may also explain the rise in S100B during physical activity [11][32][35]. Nevertheless, the contribution of S100B from lipolysis and muscular cytolysis seems to be most plausible [32][36][37]. Due to the expression of S100B in adipocytes, authors explored the relationship between serum S100B levels and body mass index (BMI) and did not find a significant association [38]. However, it is recognized that BMI is not a direct reflection of body fat, especially in athletes, for whom this parameter leads to overestimations [32].
In summary, possible extracranial sources should be taken into consideration when assessing S100B levels in mTBI patients, especially in Black individuals, patients with bone fractures and athletes.
2. S100B as a Routine Clinical Biomarker for Management of Mild Traumatic Brain Injury
2.1. Routine S100B Protein Assay
S100B is a reliable biomarker, relatively unaffected by hemolysis and storage conditions, giving it appeal for use as a clinical biomarker. Serum levels remain stable for up to 8 h at room temperature and 48 h at between 2–8 °C [39]. Erythrocytes do not contain any S100B protein, thus confirming the absence of hemolytic interference in serum S100B assays, in contrast to other biomarkers such as neuron specific enolase [40]. Note that S100B is expressed in certain lymphocyte subpopulations, implying a strict adherence to pre-analytical recommendations concerning cell separation (more especially when the samples are frozen before measurement).
In routine clinical practice, the Cobas® (Roche Diagnostics, Penzberg, Germany) and the Liaison XL® (DiaSorin, Sangtec, Saluggia, Italy) automated immunoassays are the most frequently used systems. More recently, BioMérieux also developed an automated prototype immunoassay (Vidas® 3 analyzer, bioMérieux, Marcy l’étoile, France) for serum S100B measurement without final commercialization [39]. Note that SNIBE analyzers also propose the S100B measurement. In comparison to ELISA assays, automated assays provide better analytical performance with regard to precision, linearity and accuracy, and they seem to be a preferable option for S100B determination in clinical settings [27][41]. For the two automated assays (Cobas®, Liaison XL®, DiaSorin S.p.A., Saluggia, Italy), S100B cut-off values announced by manufacturers are 0.10 and 0.15 µg/L, respectively [42]. However, the commonly accepted threshold in the management of adult patients with mTBI is 0.10 µg/L, due to the very important usage of Cobas® in international publications [43]. Finally, the results differ depending on the antibodies and the type of luminescence measurement. The two automated measurements are not interchangeable, and the use of the same method is required for the monitoring of patients. The homogeneity of the results should be improved with the international standardization [27][42].
2.2. Addition of S100B to Guidelines in General Population
In recent years, there has been increasing interest in the identification and validation of brain biomarkers in clinical routine, and the utility of blood S100B as a brain injury marker has been documented in multiple contexts such as with circulatory arrest, stroke and TBI [44][45][46]. The protein is also associated with neurodegenerative diseases such as Alzheimer’s disease [47]. Most importantly, the protein has emerged as the most promising as a biomarker of mTBI. The potential of S100B to safely reduce CT scans was first demonstrated in a large cohort of adults with mTBI (n = 1309 patients) [48]. Since then, many observational studies have confirmed the usefulness of measuring blood S100B for the exclusion of an intracranial hemorrhage in mTBI patients [49][50][51][52]. In this context, the Scandinavian guidelines were the first mTBI clinical decision rules to include the measurement of serum S100B (Figure 1A) [53][54]. The addition of S100B measurement to the guidelines allowed a one-third reduction in unnecessary CT scans, resulting in a financial saving of approximately €39 to €71 on the cost of care per patient [55]. In a meta-analysis, Undén and Romner confirmed that low serum S100B levels (<0.10 µg/L using Cobas®) accurately predicted normal CT findings after mTBI in adults, provided that the sample is collected within 3 h of injury [43]. In these conditions, the sensitivity of S100B to rule out the presence of intracranial lesions was excellent (negative predictive value ~100%), with a 30% specificity [43]. Since September 2022, the French Society for Emergency Medicine (SFMU) recommends the serum measurement of S100B (sampling within 3 h post injury) for adult patients with mTBI requiring a CT scan and presenting a medium risk of complications of intracranial lesions (Figure 1B) [56]. A recent interventional study, based on 1449 patients (the largest published cohort to date), validated the inclusion of serum S100B measurement into the SFMU’s guidelines, highlighting a theoretical reduction in the number of CT scans by 32%, with a negative predictive value of 99.6% [57]. In this study, only two S100B false negatives were reported. The intracerebral lesions observed for the two patients were not progressive, meaning that they did not get worse over time and did not require neurosurgical intervention.
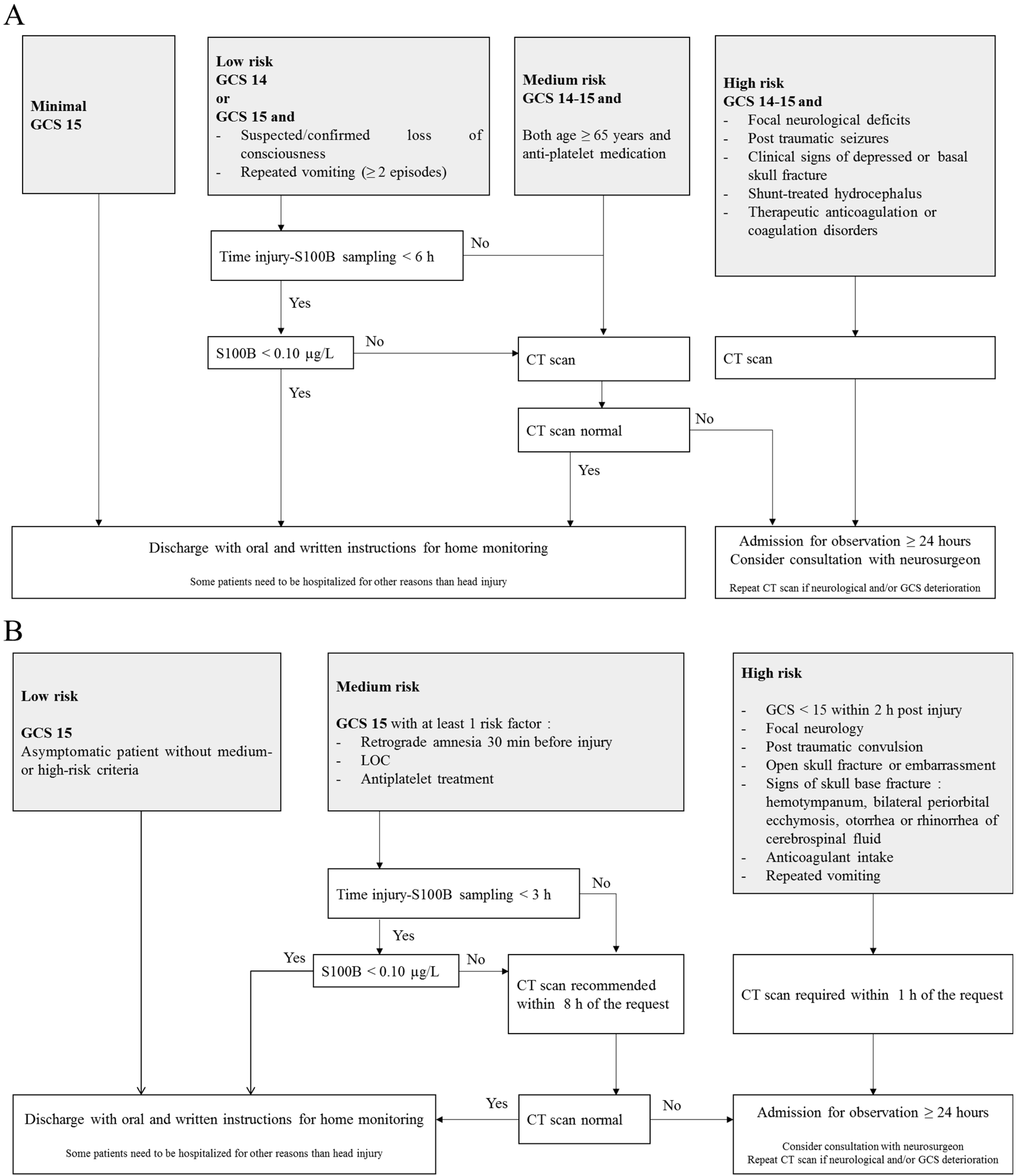
Figure 1. Scandinavian (A) and French guidelines (B) for the management of patients with mild traumatic brain injury. CT scan: Computed tomography scan; GCS: Glasgow Coma Scale; LOC: Loss of Consciousness.
The French guidelines recommend a maximum delay of 3 h between trauma and blood sampling [56] rather than the 6 h suggested by the Scandinavian guidelines [53]. Because of the short half-life of S100B, the sensitivity of the biomarker could be affected by the sampling time. In their study, Laribi et al. compared S100B concentrations measured at 3 h and 6 h post-injury and found a better sensitivity with the 3 h strategy [49]. In a previous meta-analysis based on individual data from 373 children, the researchers highlighted a sensitivity of only 90% (delay <6 h) against 97% in children whose sampling time was ≤3 h [58]. In their meta-analysis, Undén and Romner also considered that S100B should be measured within 3 h of injury [43]. Therefore, in order to avoid missing patients with intracerebral lesions in CT scans, a delay of less than 3 h would be more reliable [43][48].
To date, the evidence of the clinical utility of S100B in children is considered too low, and the biomarker is not part of the Scandinavian guidelines for the management of pediatric mTBI. In children, S100B may constitute an additional tool for the identification of low-risk patients, and it is still an area of active research.
2.3. S100B Specificities in the Pediatric Population
In adults, S100B concentrations did not differ according to age and sex [59], with a consensual threshold of 0.10 µg/L, although different thresholds could be proposed for patients over 65 years of age (see paragraph 3.4). Many studies reported higher S100B values in children (when compared to adults) [58][60]. In children, the biomarker’s concentrations are higher at birth and then gradually decrease during the first two years of life. A study of pediatric reference ranges using a Cobas® analyzer determined in a large pediatric cohort identified three age categories with decreasing S100B levels (4–9, 10–24 and >24 months) of 0.35 μg/L, 0.23 μg/L and 0.18 μg/L, respectively [61]. More recently, Simon-Pimmel et al. provided reference ranges for infants aged 0 to 4 months, with an upper reference value of 0.51 µg/L [62]. This high value could be explained by several reasons (Figure 2). Vaginal delivery may lead to brain injury, especially in cases of prolonged labor and delivery [63], when compared to planned caesarean deliveries [64]. Another likely factor influencing S100B elevation is a difference in the permeability of the BBB and cerebral circulation. Moreover, these higher values might reflect the implication of S100B in brain maturation. These data are consistent with the neurotrophic effects of the protein at physiological concentrations, with the protein stimulating neurite outgrowth and regulating neurons survival [23]. In this sense, Bouvier et al. found a significant correlation between serum S100B concentrations and head circumference, defined using the equation [27]: S100B value (μg/L) = −1.884 × head circumference (meters) + 1.0455 (r² = 0.93).
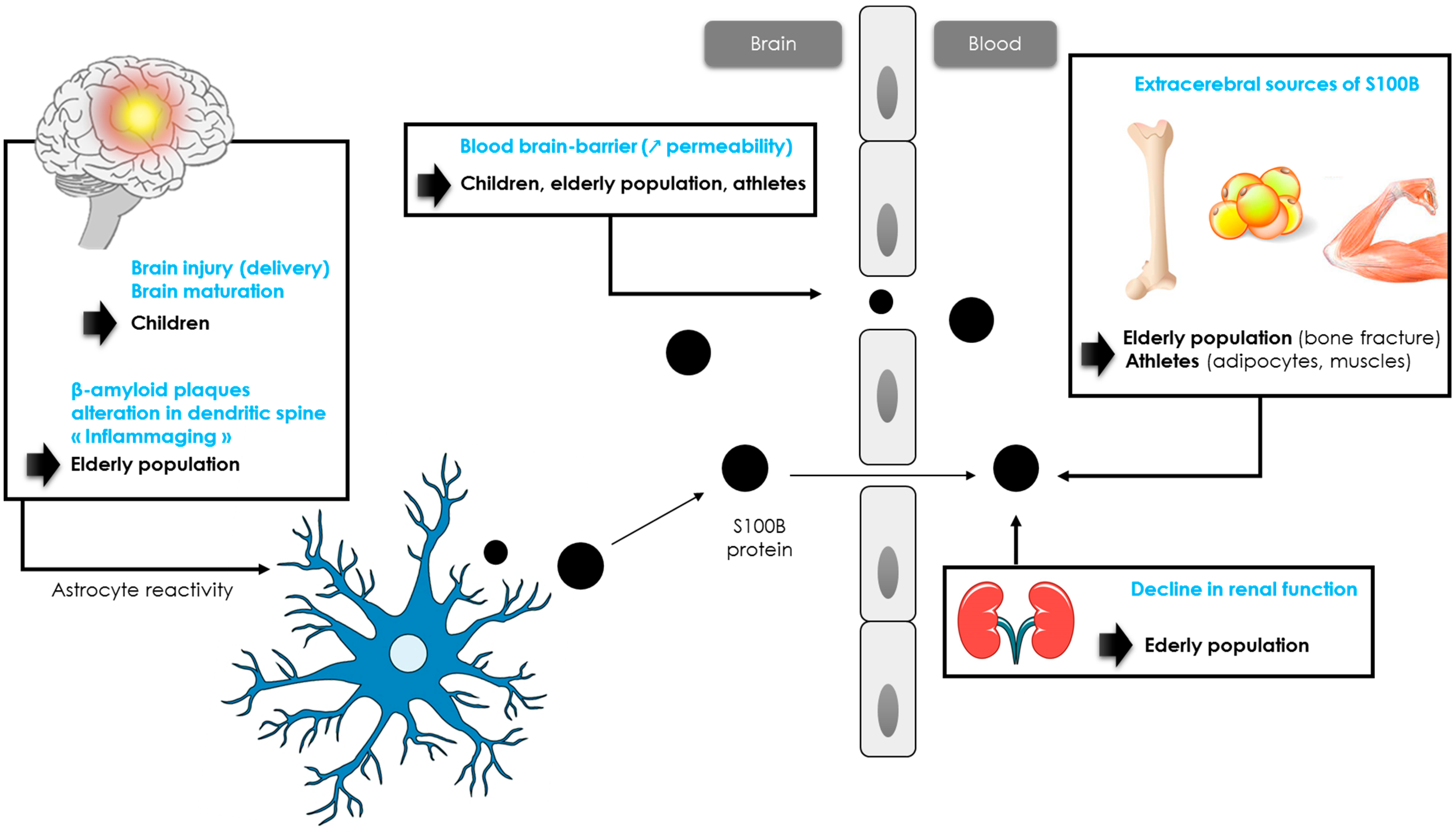
Figure 2. Hypotheses of S100B protein increase in children, athletes and elderly population.
2.4. S100B Specificities in the Elderly Population
While children have higher levels of S100B concentrations than adults, levels of S100B in older patients following mTBI are less known. In 2013, Calcagnile et al. showed that the usefulness of S100B measurement in elderly patients may be limited by a very low specificity, reflecting a smaller decrease in the number of CT scans performed [66]. Similar results were observed in a cohort of 1449 patients including 504 patients over 65 years old [57]. In this cohort, the threshold of 0.10 µg/L resulted in a 33% reduction in CT scans performed in adults versus 19% in older patients [57]. Recently, the researchers have confirmed that S100B levels were considerably affected by aging in a larger cohort of patients ≥65 years old suffering from mTBI with a medium risk of intracranial lesions [67]. As an adjustment of the S100B level was necessary in older patients, the researchers proposed the use of a new 0.15 µg/L threshold for the Cobas® analyzer in the routine management of patients aged between 80–90 years [67]. This threshold helped achieve a reduction in CT scans in the 80–90 years category similar to that in adult patients (~33%) [67]. In addition, for patients over 90 years old, the researchers do not recommend the measurement of S100B. The reduction in the number of scans allowed is considerably hampered despite the use of an age-adapted threshold [67]. Several hypotheses may be formulated to explain the increase in blood S100B with aging (Figure 2). In healthy humans, an increase in BBB permeability is observed with aging, resulting in an increase in S100B blood concentrations [68]. Another hypothesis concerns an increase in β-amyloid plaques promoting S100B synthesis [69][70]. Alterations in dendrite architecture, including changes in branching complexity, reduction in branch length, along with changes in spine morphology and reduction in spine number, may also contribute to the increase in the biomarker’s concentrations [71][72]. These alterations would trigger an activation of astrocytes, resulting in a release of trophic factors such as S100B to stimulate the regrowth of dendrites [73][74][75]. In addition, neuroinflammation associated with the physiological process of aging (“inflammaging”) would also activate astrocytes cells, leading to a release of astrocytic biomarkers such as GFAP or S100B [74][75][76]. While astrocyte activation may first be neuroprotective during normal aging, experimental data from selected central nervous system pathologies suggest that if not resolved in time, reactive gliosis can exert inhibitory effects on neuroplasticity and CNS regeneration [77]. These data are consistent with the effects of S100B protein. Nanomolar concentrations of S100B exert neurotrophic effects by stimulating neurite outgrowth and regulating the survival of neurons, while micromolar concentrations are neurotoxic [78]. Further studies are needed to better understand the role of each mechanism.
2.5. S100B Specificities in the Athletic Population
It is known that physical exercise results in a brief increase in S100B levels through extra-cerebral synthesis and/or increased BBB permeability (Figure 2) [32]. Indeed, many studies reported higher serum S100B levels after intense exercise, such as running or swimming [79][80][81][82][83]. In one study, S100B increased simultaneously to creatine kinase and myoglobin, suggesting the potential value of S100B as a biomarker of acute muscle damage after running [79]. Although serum S100B concentration has been shown to rise in relation to exercise alone, the S100B increase has been described as higher in response to the number of contacts in many sports such as hockey, American football or rugby [84][85][86][87]. Indeed, in these sports, athletes are exposed to impacts, often repetitive to the head and that do not necessarily cause signs of concussion [88]. In competitive elite soccer, serum concentrations of S100B were found to be significantly correlated to the number of headers [89]. In American football, it has been shown that blood S100B levels were elevated in post-game measures compared with the respective pre-game values. An increase in the frequency and magnitude of head impacts, without a concussion being detected, resulted in the largest acute changes in S100B plasma levels [88][90][91]. In professional rugby players, a significant increase in S100B concentration was reported within 2 h following a game (without concussion), and this increase was correlated with the number of body collisions during a match [85][92]. Since the authors did not observe a significant correlation between S100B and creatine kinase, the increase in S100B would most likely be related to sub-concussive head impacts [90][92].
2.6. Anti-S100B Antibodies, a Complementary Biomarker?
Functional BBB changes following TBI cause potential S100B protein to enter the peripheral bloodstream as «foreigner», leading to the initiation of an autoimmune response and the development of S100B autoantibodies [93][94][95]. In addition to S100B, anti-S100B autoantibodies may serve as blood-based biomarkers for brain injury, although the evidence is sparse [93]. A study conducted in children reported high levels of anti-S100B autoantibodies in the first days after severe TBI indicating failure of compensatory-adaptive immunological mechanisms and high permeability of the BBB, which are poor prognostic signs in this context [96]. Autoimmunity triggered following GFAP release into the bloodstream has also been reported in human TBI. A study including TBI patients showed an average 3.77-fold increase in anti-GFAP autoantibody levels by day 7–10 post-injury. This increase in autoantibody levels was negatively correlated with outcome at 6 months [97]. In another study, elevated anti-S100B antibodies have been observed in football players with repeated sub-concussive episodes characterized by BBB disruption. Serum levels of S100B auto-antibodies also predicted persistence of diffusion tensor imaging scan abnormalities which in turn correlated with cognitive changes [98]. In the context of TBI, the measurement of anti-S100B along with S100B represents a promising approach but requires further investigations to establish the diagnostic value of combining both in a model for concussion screening. Note that the presence of macro-analytes (circulating conjugates of analytes with immunoglobulins) is a well-known source of interference in immunoassays. Macro-analytes are high molecular weight conjugates that are measurable through the available immunoassays despite being biologically inactive. The potential analytical interference of S100B/IgG autoantibody complex, referred to as “macro-S100B”, in the S100B assay should be investigated by polyethylene glycol (PEG) precipitation and gel filtration chromatography [99]. In principle, polyethylene glycol precipitation may be applied to any immunoassay in which a macro-complex interference is suspected, this process has been extensively described in the assessment of macroprolactinemia [100]. Overall, this interference would result in an overestimation of S100B levels and may lead to a decrease in the specificity of the biomarker when screening for intracranial lesions in the management of mTBI patients. In professional athletes, who are prone to these autoantibodies, “macro-S100B” may lead to the mismanagement of the players.
This entry is adapted from the peer-reviewed paper 10.3390/ijms24076602
References
- Moore, B.W.; Mc Gregor, D. Chromatographic and Electrophoretic Fractionation of Soluble Proteins of Brain and Liver. J. Biol. Chem. 1965, 240, 1647–1653.
- Moore, B.W. A Soluble Protein Characteristic of the Nervous System. Biochem. Biophys. Res. Commun. 1965, 19, 739–744.
- Donato, R. S100: A multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int. J. Biochem. Cell Biol. 2001, 33, 637–668.
- Dempsey, B.R.; Shaw, G.S. Identification of Calcium-Independent and Calcium-Enhanced Binding between S100B and the Dopamine D2 Receptor. Biochemistry 2011, 50, 9056–9065.
- Beaudeux, J.-L. S100B protein: A novel biomarker for the diagnosis of head injury. Ann. Pharm. Françaises 2009, 67, 187–194.
- Donato, R.; Sorci, G.; Riuzzi, F.; Arcuri, C.; Bianchi, R.; Brozzi, F.; Tubaro, C.; Giambanco, I. S100B’s double life: Intracellular regulator and extracellular signal. Biochim. Biophys. Acta BBA Mol. Cell Res. 2009, 1793, 1008–1022.
- Thelin, E.P.; Nelson, D.W.; Bellander, B.-M. A review of the clinical utility of serum S100B protein levels in the assessment of traumatic brain injury. Acta Neurochir. 2017, 159, 209–225.
- Steiner, J.; Bernstein, H.-G.; Bielau, H.; Berndt, A.; Brisch, R.; Mawrin, C.; Keilhoff, G.; Bogerts, B. Evidence for a wide extra-astrocytic distribution of S100B in human brain. BMC Neurosci. 2007, 8, 2.
- Beaudeux, J.; Roche, S.; Puyssabet, L.; Foglietti, M.-J. Physiologie de la protéine S-100β et apport de son dosage dans les pathologies neurologiques. Immuno-Anal. Biol. Spéc. 2001, 16, 143–148.
- Petzold, A.; Keir, G.; Lim, D.; Smith, M.; Thompson, E. Cerebrospinal fluid (CSF) and serum S100B: Release and wash-out pattern. Brain Res. Bull. 2003, 61, 281–285.
- Kleindienst, A.; Ross Bullock, M. A Critical Analysis of the Role of the Neurotrophic Protein S100B in Acute Brain Injury. J. Neurotrauma 2006, 23, 1185–1200.
- Kanner, A.A.; Marchi, N.; Fazio, V.; Mayberg, M.; Koltz, M.T.; Siomin, V.; Stevens, G.H.J.; Masaryk, T.; Ayumar, B.; Vogelbaum, M.A.; et al. Serum S100?: A noninvasive marker of blood-brain barrier function and brain lesions. Cancer 2003, 97, 2806–2813.
- Kapural, M.; Krizanac-Bengez, L.; Barnett, G.; Perl, J.; Masaryk, T.; Apollo, D.; Rasmussen, P.; Mayberg, M.R.; Janigro, D. Serum S-100beta as a possible marker of blood-brain barrier disruption. Brain Res. 2002, 940, 102–104.
- Marchi, N.; Fazio, V.; Cucullo, L.; Kight, K.; Masaryk, T.; Barnett, G.; Volgelbaum, M.; Kinter, M.; Rasmussen, P.; Mayberg, M.R.; et al. Serum Transthyretin Monomer as a Possible Marker of Blood-to-CSF Barrier Disruption. J. Neurosci. 2003, 23, 1949–1955.
- Kleindienst, A.; Schmidt, C.; Parsch, H.; Emtmann, I.; Xu, Y.; Buchfelder, M. The Passage of S100B from Brain to Blood Is Not SpecificallyRelated to the Blood-Brain Barrier Integrity. Cardiovasc. Psychiatry Neurol. 2010, 2010, 801295.
- Ferrara, M.; Bertozzi, G.; Volonnino, G.; Di Fazio, N.; Frati, P.; Cipolloni, L.; La Russa, R.; Fineschi, V. Glymphatic System a Window on TBI Pathophysiology: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 9138.
- Li, J.P.; Lu, L.; Wang, L.J.; Zhang, F.R.; Shen, W.F. Increased serum levels of S100B are related to the severity of cardiac dysfunction, renal insufficiency and major cardiac events in patients with chronic heart failure. Clin. Biochem. 2011, 44, 984–988.
- Gross, S.; Homan van Der Heide, J.J.J.; van Son, W.J.; Gans, R.O.B.; Foell, D.; Navis, G.; Bakker, S.J.L. Body Mass Index and Creatinine Clearance Are Associated with Steady-State Serum Concentrations of the Cell Damage Marker S100B in Renal Transplant Recipients. Med. Sci. Monit. 2010, 16, CR318–CR324.
- Molina, R.; Navarro, J.; Filella, X.; Castel, T.; Ballesta, A.M. S-100 Protein Serum Levels in Patients with Benign and Malignant Diseases: False-Positive Results Related to Liver and Renal Function. Tumor Biol. 2002, 23, 39–44.
- Jönsson, H.; Johnsson, P.; Höglund, P.; Alling, C.; Blomquist, S. Elimination of S100B and renal function after cardiac surgery. J. Cardiothorac. Vasc. Anesth. 2000, 14, 698–701.
- Ghanem, G.; Loir, B.; Morandini, R.; Sales, F.; Lienard, D.; Eggermont, A.; Lejeune, F. On the release and half-life of S100B protein in the peripheral blood of melanoma patients. Int. J. Cancer 2001, 94, 586–590.
- Townend, W.; Dibble, C.; Abid, K.; Vail, A.; Sherwood, R.; Lecky, F. Rapid elimination of protein S-100B from serum after minor head trauma. J. Neurotrauma 2006, 23, 149–155.
- Zimmer, D.B.; Cornwall, E.H.; Landar, A.; Song, W. The S100 protein family: History, function, and expression. Brain Res. Bull. 1995, 37, 417–429.
- Haimoto, H.; Hosoda, S.; Kato, K. Differential Distribution of Immunoreactive S100-Alpha and S100-Beta Proteins in Normal Nonnervous Human Tissues. Lab. Investig. 1987, 57, 489–498.
- Semba, R.; Kato, K.; Isobe, T.; Kashiwamata, S. Purification of S-100a0 Protein from Rat Kidney. Brain Res. 1987, 401, 9–13.
- Dadas, A.; Washington, J.; Marchi, N.; Janigro, D. Improving the clinical management of traumatic brain injury through the pharmacokinetic modeling of peripheral blood biomarkers. Fluids Barriers CNS 2016, 13, 21.
- Bouvier, D.; Duret, T.; Rouzaire, P.; Jabaudon, M.; Rouzaire, M.; Nourrisson, C.; Bourgne, C.; Pereira, B.; Evrard, B.; Sapin, V. Preanalytical, analytical, gestational and pediatric aspects of the S100B immuno-assays. Clin. Chem. Lab. Med. 2016, 54, 833–842.
- Ben Abdesselam, O.; Vally, J.; Adem, C.; Foglietti, M.-J.; Beaudeux, J.-L. Reference Values for Serum S-100B Protein Depend on the Race of Individuals. Clin. Chem. 2003, 49, 836–837.
- Mussack, T.; Kirchhoff, C.; Buhmann, S.; Biberthaler, P.; Ladurner, R.; Gippner-Steppert, C.; Mutschler, W.; Jochum, M. Significance of Elecsys® S100 immunoassay for real-time assessment of traumatic brain damage in multiple trauma patients. Clin. Chem. Lab. Med. 2006, 44, 1140–1145.
- Müller, M.; Münster, J.M.; Hautz, W.E.; Gerber, J.L.; Schefold, J.C.; Exadaktylos, A.K.; Pfortmueller, C.A. Increased S-100 B levels are associated with fractures and soft tissue injury in multiple trauma patients. Injury 2020, 51, 812–818.
- Anderson, R.E.; Hansson, L.O.; Nilsson, O.; Dijlai-Merzoug, R.; Settergren, G. High serum S100B levels for trauma patients without head injuries. Neurosurgery 2001, 48, 1255–1258.
- Schulte, S.; Podlog, L.W.; Hamson-Utley, J.J.; Strathmann, F.G.; Strüder, H.K. A systematic review of the biomarker S100B: Implications for sport-related concussion management. J. Athl. Train. 2014, 49, 830–850.
- Michetti, F.; Bruschettini, M.; Frigiola, A.; Abella, R.; Giamberti, A.; Marchese, N.; Mangraviti, S.; Melioli, G.; Baldari, A.; Bruschettini, P.; et al. Saliva S100B in professional sportsmen: High levels at resting conditions and increased after vigorous physical activity. Clin. Biochem. 2011, 44, 245–247.
- Stålnacke, B.M.; Tegner, Y.; Sojka, P. Playing ice hockey and basketball increases serum levels of S-100B in elite players: A pilot study. Clin. J. Sport Med. 2003, 13, 292–302.
- Sharma, H.; Westman, J.; Navarro, J.C.; Dey, P.; Nyberg, F. Probable involvement of serotonin in the increased permeability of the blood—Brain barrier by forced swimming. An experimental study using Evans blue and 131I-sodium tracers in the rat. Behav. Brain Res. 1995, 72, 189–196.
- Hasselblatt, M.; Mooren, F.C.; von Ahsen, N.; Keyvani, K.; Fromme, A.; Schwarze-Eicker, K.; Senner, V.; Paulus, W. Serum S100beta Increases in Marathon Runners Reflect Extracranial Release Rather than Glial Damage. Neurology 2004, 62, 1634–1636.
- Stocchero, C.M.A.; Muller, A.P.; de Oliveira, Á.R.; Portela, L.V. A Proteína S100B e o exercício físico. Rev. Bras. Cineantropom. Desempenho Hum. 2010, 12, 77–81.
- Pham, N.; Fazio, V.; Cucullo, L.; Teng, Q.; Biberthaler, P.; Bazarian, J.J.; Janigro, D. Extracranial sources of S100B do not affect serum levels. PLoS ONE 2010, 5, e12691.
- Oris, C.; Chabanne, R.; Durif, J.; Kahouadji, S.; Brailova, M.; Sapin, V.; Bouvier, D. Measurement of S100B protein: Evaluation of a new prototype on a bioMérieux Vidas® 3 analyzer. Clin. Chem. Lab. Med. 2019, 57, 1177–1184.
- Beaudeux, J.; Léger, P.; Dequen, L. Influence of Hemolysis on the Measurement of S100B Protein and Neuron-Specific Enolase Plasma Concentrations during Coronary Artery Bypass Grafting. Clin. Chem. 2000, 46, 989–990.
- Smit, L.H.M.; Korse, C.M.; Bonfrer, J.M.G. Comparison of four different assays for determination of serum S-100B. Int. J. Biol. Markers 2005, 20, 34–42.
- Feriel, J.; Adamo, F.; Monneret, D.; Trehel-Tursis, V.; Favard, S.; Tsé, C.; Puybasset, L.; Bonnefont-Rousselot, D.; Imbert-Bismut, F. S100B protein concentration measurement according to two different immunoassays. Clin. Chem. Lab. Med. 2015, 53, e169–e171.
- Undén, J.; Romner, B. Can low serum levels of S100B predict normal CT findings after minor head injury in adults?: An evidence-based review and meta-analysis. J. Head Trauma Rehabil. 2010, 25, 228–240.
- Dadas, A.; Washington, J.; Diaz-Arrastia, R.; Janigro, D. Biomarkers in traumatic brain injury (TBI): A review. Neuropsychiatr. Dis. Treat. 2018, 14, 2989–3000.
- Dassan, P.; Keir, G.; Brown, M.M. Criteria for a Clinically Informative Serum Biomarker in Acute Ischaemic Stroke: A Review of S100B. Cerebrovasc. Dis. 2009, 27, 295–302.
- Bhattacharya, K.; Westaby, S.; Pillai, R.; Standing, S.J.; Johnsson, P.; Taggart, D.P. Serum S100B and hypothermic circulatory arrest in adults. Ann. Thorac. Surg. 1999, 68, 1225–1229.
- Yardan, T.; Erenler, A.K.; Baydin, A.; Aydin, K.; Cokluk, C. Usefulness of S100B Protein in Neurological Disorders. J. Pak. Med. Assoc. 2011, 61, 276–281.
- Biberthaler, P.; Linsenmeier, U.; Pfeifer, K.-J.; Kroetz, M.; Mussack, T.; Kanz, K.-G.; Hoecherl, E.F.; Jonas, F.; Marzi, I.; Leucht, P.; et al. Serum S-100B concentration provides additional information fot the indication of computed tomography in patients after minor head injury: A prospective multicenter study. Shock 2006, 25, 446–453.
- Laribi, S.; Kansao, J.; Borderie, D.; Collet, C.; Deschamps, P.; Ababsa, R.; Mouniam, L.; Got, L.; Leon, A.; Thoannes, H.; et al. S100B blood level measurement to exclude cerebral lesions after minor head injury: The multicenter STIC-S100 French study. Clin. Chem. Lab. Med. 2014, 52, 527–536.
- Ingebrigtsen, T.; Romner, B.; Marup-Jensen, S.; Dons, M.; Lundqvist, C.; Bellner, J.; Alling, C.; Børgesen, S.E. The clinical value of serum S-100 protein measurements in minor head injury: A Scandinavian multicentre study. Brain Inj. 2000, 14, 1047–1055.
- Müller, K.; Townend, W.; Biasca, N.; Undén, J.; Waterloo, K.; Romner, B.; Ingebrigtsen, T. S100B serum level predicts computed tomography findings after minor head injury. J. Trauma 2007, 62, 1452–1456.
- Bouvier, D.; Oddoze, C.; Ben Haim, D.; Moustafa, F.; Legrand, A.; Alazia, M.; Jehle, E.; Schmidt, J.; Sapin, V. Interest of S100B protein blood level determination for the management of patients with minor head trauma. Ann. Biol. Clin. 2009, 67, 425–431.
- Undén, J.; Ingebrigtsen, T.; Romner, B.; The Scandinavian Neurotrauma Committee (SNC). Scandinavian guidelines for initial management of minimal, mild and moderate head injuries in adults: An evidence and consensus-based update. BMC Med. 2013, 11, 50.
- Astrand, R.; Rosenlund, C.; Undén, J. Scandinavian guidelines for initial management of minor and moderate head trauma in children. BMC Med. 2016, 14, 33.
- Calcagnile, O.; Anell, A.; Undén, J. The addition of S100B to guidelines for management of mild head injury is potentially cost saving. BMC Neurol. 2016, 16, 200.
- Gil-Jardiné, C.; Payen, J.-F.; Bernard, R.; Bobbia, X.; Bouzat, P.; Catoire, P.; Chauvin, A.; Claessens, Y.-E.; Douay, B.; Dubucs, X. Management of Patients Suffering from Mild Traumatic Brain Injury. Société Française de Médecine d’Urgence (SFMU), Société Française d’Anesthésie et de Réanimation (SFAR). 2022. Available online: https://www.sfmu.org/upload/consensus/RPP-TCL-2022.pdf (accessed on 29 March 2023).
- Allouchery, G.; Moustafa, F.; Roubin, J.; Pereira, B.; Schmidt, J.; Raconnat, J.; Pic, D.; Sapin, V.; Bouvier, D. Clinical validation of S100B in the management of a mild traumatic brain injury: Issues from an interventional cohort of 1449 adult patients. Clin. Chem. Lab. Med. 2018, 56, 1897–1904.
- Oris, C.; Pereira, B.; Durif, J.; Simon-Pimmel, J.; Castellani, C.; Manzano, S.; Sapin, V.; Bouvier, D. The Biomarker S100B and Mild Traumatic Brain Injury: A Meta-analysis. Pediatrics 2018, 141, e20180037.
- Wiesmann, M.; Missler, U.; Gottmann, D.; Gehring, S. Plasma S-100b Protein Concentration in Healthy Adults Is Age- and Sex-Independent. Clin. Chem. 1998, 44, 1056–1058.
- Bouvier, D.; Fournier, M.; Dauphin, J.-B.; Amat, F.; Ughetto, S.; Labbé, A.; Sapin, V. Serum S100B Determination in the Management of Pediatric Mild Traumatic Brain Injury. Clin. Chem. 2012, 58, 1116–1122.
- Bouvier, D.; Castellani, C.; Fournier, M.; Dauphin, J.-B.; Ughetto, S.; Breton, M.; Labbé, A.; Weinberg, A.-M.; Sapin, V. Reference ranges for serum S100B protein during the first three years of life. Clin. Biochem. 2011, 44, 927–929.
- Simon-Pimmel, J.; Lorton, F.; Masson, D.; Bouvier, D.; Hanf, M.; Guen, C.G.-L. Reference ranges for serum S100B neuroprotein specific to infants under four months of age. Clin. Biochem. 2017, 50, 1056–1060.
- Schulpis, K.H.; Margeli, A.; Akalestos, A.; Vlachos, G.D.; Partsinevelos, G.A.; Papastamataki, M.; Antsaklis, A.; Papassotiriou, I. Effects of Mode of Delivery on Maternal-Neonatal Plasma Antioxidant Status and on Protein S100B Serum Concentrations. Scand. J. Clin. Lab. Investig. 2006, 66, 733–742.
- Wirds, J.W. S100 protein content of umbilical cord blood in healthy newborns in relation to mode of delivery. Arch. Dis. Child Fetal Neonatal Ed. 2003, 88, F67–F69.
- Simon-Pimmel, J.; Lorton, F.; Guiziou, N.; Levieux, K.; Vrignaud, B.; Masson, D.; Dupas, B.; Guen, C.G.-L. Serum S100β Neuroprotein Reduces Use of Cranial Computed Tomography in Children After Minor Head Trauma. Shock 2015, 44, 410–416.
- Calcagnile, O.; Holmén, A.; Chew, M.; Undén, J. S100B levels are affected by older age but not by alcohol intoxication following mild traumatic brain injury. Scand. J. Trauma Resusc. Emerg. Med. 2013, 21, 52.
- Oris, C.; Bouillon-Minois, J.B.; Pinguet, J.; Kahouadji, S.; Durif, J.; Meslé, V.; Pereira, B.; Schmidt, J.; Sapin, V.; Bouvier, D. Predictive Performance of Blood S100B in the Management of Patients Over 65 Years Old With Mild Traumatic Brain Injury. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 1471–1479.
- Farrall, A.J.; Wardlaw, J.M. Blood–brain barrier: Ageing and microvascular disease—Systematic review and meta-analysis. Neurobiol. Aging 2009, 30, 337–352.
- Schmitt, A.; Bertsch, T.; Henning, U.; Tost, H.; Klimke, A.; Henn, F.A.; Falkai, P. Increased serum S100B in elderly, chronic schizophrenic patients: Negative correlation with deficit symptoms. Schizophr. Res. 2005, 80, 305–313.
- Pefia, L.A.; Brecher, C.W.; Marshak, D.R. 13-Amyloid regulates gene expression of glial trophic substance S100/3 in C6 glioma and primary astrocyte cultures. Mol. Brain Res. 1995, 34, 118–126.
- Dickstein, D.L.; Kabaso, D.; Rocher, A.B.; Luebke, J.; Wearne, S.L.; Hof, P.R. Changes in the structural complexity of the aged brain. Aging Cell 2007, 6, 275–284.
- Benavides-Piccione, R.; Fernaud-Espinosa, I.; Robles, V.; Yuste, R.; DeFelipe, J. Age-Based Comparison of Human Dendritic Spine Structure Using Complete Three-Dimensional Reconstructions. Cereb. Cortex 2012, 23, 1798–1810.
- Agid, Y. Vieillissement Cérébral Ou Maladie Dégénérative; Colloque Chimie et Cerveau, 12 Novembre 2014; Fondation de La Maison de La Chimie: Paris, France, 2014.
- Rodríguez-Arellano, J.J.; Parpura, V.; Zorec, R.; Verkhratsky, A. Astrocytes in physiological aging and Alzheimer’s disease. Neuroscience 2016, 323, 170–182.
- Coleman, P.D.; Flood, D.G. Chapter 14 Dendritic Proliferation in the Aging Brain as a Compensatory Repair Mechanism. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 1986; Volume 70, pp. 227–237.
- Matias, I.; Morgado, J.; Gomes, F.C.A. Astrocyte Heterogeneity: Impact to Brain Aging and Disease. Front. Aging Neurosci. 2019, 11, 59.
- Pekny, M.; Wilhelmsson, U.; Pekna, M. The dual role of astrocyte activation and reactive gliosis. Neurosci. Lett. 2014, 565, 30–38.
- Huttunen, H.J.; Kuja-Panula, J.; Sorci, G.; Agneletti, A.L.; Donato, R.; Rauvala, H. Coregulation of Neurite Outgrowth and Cell Survival by Amphoterin and S100 Proteins through Receptor for Advanced Glycation End Products (RAGE) Activation. J. Biol. Chem. 2000, 275, 40096–40105.
- Stocchero, C.M.; Oses, J.P.; Cunha, G.S.; Martins, J.B.; Brum, L.M.; Zimmer, E.R.; Souza, D.O.; Portela, L.V.; Reischak-Oliveira, A. Serum S100B Level Increases after Running but Not Cycling Exercise. Appl. Physiol. Nutr. Metab. 2014, 39, 340–344.
- Rogatzki, M.J.; Keuler, S.A.; Harris, A.E.; Ringgenberg, S.W.; Breckenridge, R.E.; White, J.L.; Baker, J.S. Response of protein S100B to playing American football, lifting weights, and treadmill running. Scand. J. Med. Sci. Sports 2018, 28, 2505–2514.
- Jouffroy, R.; Alves, B.; Mauvieux, B.; Mallet, L.; Beaudeux, J.L.; Cottart, C.H. NSE & S100B Protein Blood Level Assessment during a Long-Distance Trail Race. Ann. Biol. Clin. 2019, 77, 532–536.
- Otto, M.; Holthusen, S.; Bahn, E.; Söhnchen, N.; Wiltfang, J.; Geese, R.; Fischer, A.; Reimers, C.D. Boxing and running lead to a rise in serum levels of S-100B protein. Int. J. Sports Med. 2000, 21, 551–555.
- Schroeder, J.; Erthel, F.; Hollander, K. Effects of Foot-Strike Patterns on Biomarkers S100 Calcium-Binding Protein B/Neuron-Specific Enolase in Running-A Pilot Study. Int. J. Sports Physiol. Perform. 2020, 15, 900–902.
- Mussack, T.; Dvorak, J.; Graf-Baumann, T.; Jochum, M. Serum S-100B protein levels in young amateur soccer players after controlled heading and normal exercise. Eur. J. Med. Res. 2003, 8, 457–464.
- O’Connell, B.; Wilson, F.; Boyle, N.; O’Dwyer, T.; Denvir, K.; Farrell, G.; Kelly, M. Effects of match play and training on circulating S100B concentration in professional rugby players. Brain Inj. 2018, 32, 1811–1816.
- Shahim, P.; Tegner, Y.; Marklund, N.; Blennow, K.; Zetterberg, H. Neurofilament light and tau as blood biomarkers for sports-related concussion. Neurology 2018, 90, e1780–e1788.
- Meier, T.B.; Nelson, L.D.; Huber, D.; Bazarian, J.J.; Hayes, R.L.; McCrea, M.A. Prospective Assessment of Acute Blood Markers of Brain Injury in Sport-Related Concussion. J. Neurotrauma 2017, 34, 3134–3142.
- Kawata, K.; Rubin, L.H.; Takahagi, M.; Lee, J.H.; Sim, T.; Szwanki, V.; Bellamy, A.; Tierney, R.; Langford, D. Subconcussive Impact-Dependent Increase in Plasma S100β Levels in Collegiate Football Players. J. Neurotrauma 2017, 34, 2254–2260.
- Stålnacke, B.-M.; Tegner, Y.; Sojka, P. Playing Soccer Increases Serum Concentrations of the Biochemical Markers of Brain Damage S-100B and Neuron-Specific Enolase in Elite Players: A Pilot Study. Brain Inj. 2004, 18, 899–909.
- Zonner, S.W.; Ejima, K.; Bevilacqua, Z.W.; Huibregtse, M.E.; Charleston, C.; Fulgar, C.; Kawata, K. Association of Increased Serum S100B Levels With High School Football Subconcussive Head Impacts. Front. Neurol. 2019, 10, 327.
- Rubin, L.H.; Tierney, R.; Kawata, K.; Wesley, L.; Lee, J.H.; Blennow, K.; Zetterberg, H.; Langford, D. NFL Blood Levels Are Moderated by Subconcussive Impacts in a Cohort of College Football Players. Brain Inj. 2019, 33, 456–462.
- Bouvier, D.; Duret, T.; Abbot, M.; Stiernon, T.; Pereira, B.; Coste, A.; Chazal, J.; Sapin, V. Utility of S100B Serum Level for the Determination of Concussion in Male Rugby Players. Sports Med. 2016, 47, 781–789.
- Huibregtse, M.E.; Bazarian, J.J.; Shultz, S.R.; Kawata, K. The biological significance and clinical utility of emerging blood biomarkers for traumatic brain injury. Neurosci. Biobehav. Rev. 2021, 130, 433–447.
- Yang, Z.; Zhu, T.; Weissman, A.S.; Jaalouk, E.; Rathore, D.S.; Romo, P.; Shi, Y.; Wagner, A.K.; Wang, K.K.W. Autoimmunity and Traumatic Brain Injury. Curr. Phys. Med. Rehabil. Rep. 2017, 5, 22–29.
- Bargerstock, E.; Puvenna, V.; Iffland, P.; Falcone, T.; Hossain, M.; Vetter, S.; Man, S.; Dickstein, L.; Marchi, N.; Ghosh, C.; et al. Is Peripheral Immunity Regulated by Blood-Brain Barrier Permeability Changes? PLoS ONE 2014, 9, e101477.
- Pinelis, V.G.; Sorokina, E.G.; Semenova, J.B.; Karaseva, O.V.; Mescheryakov, S.V.; Chernisheva, T.A.; Arsenieva, E.N.; Roshal, L.M. Biomarkers in children with traumatic brain injury. Zh. Nevrol. Psikhiatr. Im. S S Korsakova 2015, 115, 66–72.
- Zhang, Z.; Zoltewicz, J.S.; Mondello, S.; Newsom, K.J.; Yang, Z.; Yang, B.; Kobeissy, F.; Guingab, J.; Glushakova, O.; Robicsek, S.; et al. Human Traumatic Brain Injury Induces Autoantibody Response against Glial Fibrillary Acidic Protein and Its Breakdown Products. PLoS ONE 2014, 9, e92698.
- Marchi, N.; Bazarian, J.J.; Puvenna, V.; Janigro, M.; Ghosh, C.; Zhong, J.; Zhu, T.; Blackman, E.; Stewart, D.; Ellis, J.; et al. Consequences of repeated blood-brain barrier disruption in football players. PLoS ONE 2013, 8, e56805.
- Gessl, A.; Blueml, S.; Bieglmayer, C.; Marculescu, R. Anti-Ruthenium Antibodies Mimic Macro-TSH in Electrochemiluminescent Immunoassay. Clin. Chem. Lab. Med. 2014, 52, 1589–1594.
- Sturgeon, C.M.; Viljoen, A. Analytical error and interference in immunoassay: Minimizing risk. Ann. Clin. Biochem. Int. J. Biochem. Lab. Med. 2011, 48, 418–432.
This entry is offline, you can click here to edit this entry!
