Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Others
Three-dimensional (3D) bioprinting has emerged as a promising scaffold fabrication strategy for tissue engineering with excellent control over scaffold geometry and microstructure. Nanobiomaterials as bioinks play a key role in manipulating the cellular microenvironment to alter its growth and development.
- 3D bioprinting
- nanobiomaterials
- nanotechnology
1. Introduction
Tissue engineering (TE) is an interdisciplinary field that applies the principles of engineering and life sciences toward the development of biological substitutes that restore, maintain, or improve tissue functions [1]. The three elements of tissue engineering are scaffolds, cells, and signal molecules, of which scaffolds can be called “beating hearts” [2]. It is a 3D structure of an artificial extracellular matrix used in tissue engineering. It provides a temporary environment for cell adhesion, growth, reproduction, oxygen diffusion, nutrient transport, and waste removal, and minimizes inflammation and toxicity [3,4]. It can also be biodegraded at a controlled rate to support the formation of new tissues [5]. Based on the above, the scaffold needs to have (i) mechanical strength that can resist external pressure, maintain the original shape and integrity of the tissue, (ii) promote cell adhesion and proliferation, non-toxic, no inflammation reaction, and biocompatibility, (iii) appropriate degradation rate and other properties. Therefore, the chemical composition, physical structure, and biological functional parts are all important for tissue engineering scaffolds [6,7,8].
Considering the complexity of the human body, it is critical to select suitable biomaterials as scaffold media in tissue engineering. Currently, polymers (including natural polymers, synthetic polymers, and polymer-derived materials) have dominated tissue engineering scaffolds [9]. In parallel, nanotechnology is a broad, versatile, and diverse area and can be defined as the production, characterization, and application of different materials, devices, or systems while maintaining control of the shape and size on a nanometric scale [10]. Nanotechnology is excellent in constructing three-dimensional scaffolds suitable for various tissue engineering applications because its nanoscale size allows materials to support and guide cell activity at the cellular and subcellular levels. Therefore, nanofibers, nanoparticles, nanocrystals, and other types of nanomaterials are increasingly introduced as powerful tools for biofunctionalized scaffold materials to process and control the three-dimensional shape and geometry of scaffolds [11,12].
Choosing production technology depends on the specific requirements of the bracket, related materials, and machine constraints [13]. Various fabrication methods, such as freeze-drying, phase separation, gas foaming, particle leaching, and solvent casting, have been developed to produce tissue scaffolds in recent years. However, many manufacturing methods are traditional, not fully mimicking the inherent structure of the tissue or using organic solvents, and they are difficult to support cell growth. Among the technologies, 3D bioprinting is a promising strategy for fabricating scaffolds for tissue engineering. Based on an on-demand 3D model, it locates and assembles biomaterials (living cells can also be mixed in customized biomaterials) to manufacture biomedical products such as artificially implanted stents, tissues, and organs through computer-aided design software layered discretization and Computer Numerical Control molding methods. This technology not only has excellent control over the geometry and microstructure of the scaffold but also enables living cells and growth factors to be integrated into the scaffold during the manufacturing process [14].
2. Materials for 3D Bioprinting
Nanotechnology is defined as the technological progress of basic nanoscale materials that can be applied in daily life [15,16]. As biomaterial-cell interactions are key to cell viability, proliferation, and differentiation, it is necessary to consider the characteristics of biomaterials, such as non-toxicity, good biocompatibility, and no immune and foreign body reaction [17]. In the process of using 3D printing technology to construct tissue engineering scaffolds, the use of nanomaterials enhances shape fidelity and printability apart from endowing plentiful biological functions to the bioinks [18]. In this part, 3D scaffolds are divided into synthetic polymer scaffolds, natural polymer scaffolds, and polymer derivative scaffolds according to the source of manufacturing materials.
2.1. Natural Polymer
Natural biopolymers have resurged over the past few decades as primary bioactive substances [19]. Biofunctional molecules which ensure bioactivity, biomimetic nature, and natural restructuring are typically found in such polymers. Natural polymers are macromolecular compounds existing in organisms, including chitosan [20], cellulose [21], alginate [22], and collagen [23] (Figure 1).
2.1.1. Chitosan
The raw material source of chitosan is the exoskeleton of marine crustaceans, such as crabs, lobsters, shrimps, and krill, which has low toxicity, a changeable structure, abundant functional groups, and can be processed into various shapes and sizes by 3D printing [24]. Although it has many advantages in tissue engineering, low strength limits the application of chitosan [25]. Nano-sized materials were added to chitosan as fillers and dispersed in the whole matrix to resolve the problem of structural defects. Sadeghianmaryan et al. [26] developed a chitosan/sodium alginate/nano-hydroxyapatite scaffold by impregnating sodium alginate and nano-hydroxyapatite (nHA) into a printed chitosan scaffold with 3D printing technology. The compression test showed that the addition of nHA increased the elastic modulus of the scaffold, and the live/dead cell analysis showed that nHA enhanced cell viability and attachment. Chen et al. [27] developed a 3D-printable chitosan cryogel using difunctional polyurethane nanoparticles as the crosslinker. The cryogel was printed on a liquid cryodeposition manufacturing platform by a 3D printer and had similar properties to bulk cryogel, such as high compressibility, elastic recovery, and water absorption (≈3200%). The cell experiments showed that the 3D-printed chitosan cryogel scaffold was injectable and shape-restorable, which could provide good mechanical integrity for the proliferation of human adipose-derived adult stem cells and cartilage differentiation. Zhang et al. [28] developed chitosan/silk composite scaffolds using silk nanoparticles, silk microfibers, and silk nanofibers by an extrusion-based 3D printing method (Figure 1A). The surface of the scaffolds is hydrophilic, and the fabricated scaffolds can support stable cell growth. Among them, the use of silk nanoparticle geometry had a significant effect on the mechanical properties, and the 3D-printed scaffolds prepared with silk nanofiber fillers have the highest fibroblast proliferation rate and more elongated morphology. These findings indicate the potential of nanomaterials as ink fillers.
2.1.2. Cellulose
Cellulose is a kind of β-1,4-linked glucopyranoside polymer, which is environment-friendly, renewable, biodegradable, biocompatible, non-toxic, and can be covalently linked to many bioactive molecules [29]. Cellulose nanofiber (CNF) can be obtained in two ways. The first method is to turn plant cellulose into CNF. The second is to synthesize BC nanofibers through bacterial fermentation of sugar [30]. Nanocellulose has a large surface area and high mechanical strength, which is mostly used for complex scaffold fabrication and to increase the stiffness of biomaterials [31]. Samfors et al. [32] developed a method to create channel structures within nanoporous bacterial cellulose (BNC) scaffolds that used a 3D printer to interconnect macroporosity and vessel-like lumens. This structure enabled endothelial cells to be arranged to form a vascular-mimicking network, and the microscopic nano-morphology of the scaffold increased oxygen and nutrient diffusion, which could be used to simulate the vascular system or other tubular tissues. Xu et al. [33] developed a 3D printing method to form double-crosslinked nanocellulose hydrogels and explored optimized printing and geometric design parameters to further adjust the stiffness of the printed scaffolds so that the mechanical strength of the scaffolds could be well-tuned in the 3 to 8 kPa range and promoted cell proliferation when stiffness increased within this range (Figure 1B). This correlation was first demonstrated by 3D-printed nanocellulose hydrogels.
2.1.3. Alginate
Alginate is non-toxic, hydrophilic, biocompatible, and biodegradable. However, bioinks composed of alginate have shear thinning properties and reduced viscosity (lower viscosity provides less shear stress, thus reducing cell damage), which can cause printing difficulties when using extrusion 3D bioprinting techniques [34]. In order to improve alginate applicability for bioink, it is often functionalized by nanomaterials to obtain better printability. Abouzeid et al. [35] used sodium alginate and polyvinyl alcohol (PVA)-grafted cellulose nanofibers to fabricate hydrogel scaffolds with 3D printing. By varying the CNF content, they investigated how the viscosity of the hydrogel changes along with the shear rate at room temperature. The results showed the increase in CNF content leads to higher viscosity values, which is related to the tight entanglement between the nanofibers. When the shear rate is increased, all the as-prepared hydrogel scaffolds exhibit shear-thinning behavior with decreasing viscosity; this may be related to the typical breakdown of the hydrogel nanofiber network at high shear rates [36]. Shang et al. [37] proposed a mixed 3D printing and electrodeposition method of calcium alginate hydrogel. The specific method is as follows: the injection syringe is connected to the traditional 3D printer, sodium alginate, and CaCO3 nanoparticles are sprayed from the injector nozzle to the conductive substrate as fillers, then the Ca2+ released from CaCO3 particles via electric pressure that makes the alginate cross-linked to form calcium alginate hydrogel. By controlling the voltage, deposition time, movement, and other parameters of the 3D printer, various 3D structured alginate gels can be formed (Figure 1C).
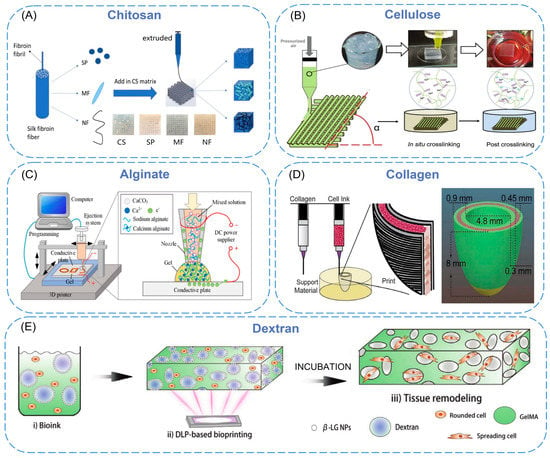
Figure 1. (A) Schematic elucidating 3D printing process of chitosan/silk composite scaffolds. (B) Illustration of 3D printing of CNF hydrogels. (C) A schematic of the developed system: a PC is used to control the movement of the 3D printer and the ejection flow rate, and a DC voltage is applied across the conductive plate (anode) and the nozzle (cathode) to trigger the electrodeposition process. (D) Schematic of dual-material printing using a collagen ink (green) and a high-concentration cell ink (pink). (E) Schematic illustration of DLP-based bioprinting of porous tissue constructs using nanoparticle-stabilized emulsion bioink.
2.1.4. Collagen
Collagen mainly exists in the human extracellular matrix (ECM) and is the most abundant protein in mammals. It is responsible for maintaining the sustainability of the three-dimensional microstructure. It has stability and high biocompatibility [40]. Li et al. [41] prepared a bioink for 3D printing by dispersing nano-hydroxyapatite and deproteinized bovine bone into collagen. They fabricated a porous scaffold using a 3D printer to obtain the bone replacement material closest to the natural bone structure and composition. By evaluating the biocompatibility, it was found that the composite material was beneficial to the proliferation and differentiation of bone marrow mesenchymal stem cells. Chou et al. [42] combined fused deposition modeling and electrospinning techniques to embed poly(d,l-lactide-co-glycolide) (PLGA) in collagen nanofibrous membranes and integrated them onto polylactic acid (PLA) bone plugs. The in vivo effects of PLGA/collagen nanofibers and PLA bolts on tendon-bone healing was investigated on a rabbit bone tunnel model by histology and tendon pullout assays. They found PLGA/collagen nanofibrous membranes triggered more tendon-osseointegration in the lateral cortex, indicating that the composite polymer could effectively promote tendon remodeling in the rabbit model. In a recent report, an elliptical left ventricular model was printed by using a dual-material printing strategy [43]. Collagen bioink was used as the structural component of the inner and outer walls to provide sufficient structural integrity and maintain the expected geometric shape (Figure 1D). The central core area combined high-density cell bioink (cardiac muscle cells derived from human stem cells, etc.) to complete synchronous contraction and directional action potential transmission.
2.1.5. Dextran
Dextran is a bacterial polysaccharide consisting essentially of α-1,6 linked glucopyranoside residues with a small percentage of α-1,3 linked residues. It has the advantages of biocompatibility, low toxicity, relatively low cost, and simple modification [44]. Wang et al. [45] proposed a dual-functional GelMAdextran aqueous two-phase emulsion bioink, simultaneously formulated with IL-4-loaded AgGNRs and MSCs. They found that the PGelDex bioinks exhibited excellent printability in DLP, conventional extrusion, and handheld extrusion bioprinting methods. Furthermore, this functional micropore-forming bioink could successfully suppress both Gram-positive and Gram-negative bacterial growth. The data also showed that the existence of IL-4 and MSCs promoted the differentiation of the M2 phenotype of macrophages, indicating that biological ink has a potential anti-inflammatory function. Tao et al. [39] produced bio-inks through the integration of GelMA solution and β-lactoglobulin (β-LG) nanoparticles/dextran solution mixture and printed tissue constructs using 3D bioprinting technology based on digital light processing (Figure 1E). After the ink is solidified, it is immersed in the culture medium to wash out the dextran so as to form pores in situ, allowing nutrients and oxygen to diffuse into the 3D-printed hydrogel construction. This mode allows precise and complex structure bioprinting. In addition, they proved that after subcutaneous implantation in nude mice, the 3D-printed porous structure trachea showed the ability to support the survival and maturation of chondrocytes and induced successful cartilage reconstruction in vivo.
2.2. Synthetic Polymer
Synthetic polymers are man-made polymers produced through chemical reactions with tunable chemical structures and physical properties. Compared to natural polymers, synthetic polymers are cheaper, strengthener, and have better functionality [46]. Some synthetic polymers are biodegradable, and these polymers can be degraded by microorganisms or biological fluids in vivo [47]. The commonly used biodegradable synthetic polymers include polylactic acid (PLA), poly(lactic-co-glycolic acid (PLGA), polycaprolactone (PCL), polyurethane (PU), and polyvinyl alcohol (PVA). However, few biodegradable synthetic polymers can meet all the needs of 3D printing of bioartificial organs. The current solution is combining these polymers with nanomaterials that are easy to be produced under controlled conditions and have strong mechanical properties [48] (Figure 2).
2.2.1. Polylactic Acid (PLA)
Polylactic acid (PLA) is a biodegradable, bioabsorbable, thermoplastic aliphatic polyester. It is derived from renewable resources, contains repeating lactic acid units, and includes both D- and L-stereoisomers or be enantiomerically pure (e.g., PLLA contains only L stereocenters) [49,50,51,52]. However, the poor mechanical properties limited its applications [53,54]. To improve its mechanical strength, various nano-additives have been added to PLA. PLA-Silk Fibroin/NGF (PS/N) scaffolds were prepared by coaxial electrospinning, and p-PS/N nanofibers were obtained by air plasma treatment [55]. During coaxial electrospinning, PLA and silk fibroin nanofibers were sprayed by two different needles to form core-shell composite nanomaterials (Figure 2A). The diameters of PS/N and p-PS/N were 221 nm and 228 nm, respectively, and the water contact angle changed from 133.60° to 0°. It showed that plasma treatment significantly improved the hydrophilicity of the scaffold without damaging the fibers. In addition, the scaffold has been shown to support the attachment and differentiation of PC12 cells and can be used as a suitable matrix for neural tissue engineering. Naghieh et al. [56] fabricated several sets of pure PLA scaffolds with different pore sizes and shapes using fused deposition modeling (FDM), then developed the scaffolds by adding nanocomposite hairless gelatin forsterite layers via electrospinning technology. The formation of apatite on the scaffold surface was investigated by immersion in simulated body fluids. The results showed that the elastic modulus of the PLA/gelatin forsterite scaffold was significantly higher than the pure scaffold (about 52%), and the calcium phosphate-like precipitate formed on the surface confirmed that the nanocomposite fibrous layer had improved scaffold bioactivity. It is expected to be used for bone tissue regeneration in the future. In the study of K. Dave [57], amphiphilic nanomaterial carbon dots (CDs) were melt-blended with polylactic acid to be extruded into filaments suitable for 3D printing, and then they used FDM technology to print porous scaffolds, which were mixed with untreated materials. In contrast, treatments were found to enhance cell adhesion, proliferation, and migration in biological environments, providing opportunities for built-in scanning and monitoring of scaffolds and cellular environments. Prasopthum et al. [58] used extrusion-based 3D printing technology to incorporate hydroxyapatite nanoparticles (nHA) into polylactic acid (PLLA) to form nanofiber chains and then successfully printed self-supporting structures with different characteristics from nanometers to centimeters at room temperature. The mechanical properties of the scaffold were improved, and the osteogenesis of bone marrow mesenchymal stem cells was promoted.
2.2.2. Poly(Lactic-co-Glycolic Acid) (PLGA)
Poly(lactic-co-glycolic acid) (PLGA) is a functional polymer organic compound, which is randomly polymerized from two monomers, lactic acid, and glycolic acid, and has adjustable biodegradability. However, the acidic microenvironment caused by its degradation products causes poor biological activity (e.g., osteoconductivity and osteoinductivity), so nano-materials are added to PLGA to improve this performance [59]. Rasoulianboroujeni et al. [60] introduced the advantages of 3D-printed PLGA/TiO2 nanocomposite scaffolds in re-bone tissue engineering. Compared with pure PLGA, the addition of TiO2 nanoparticles to PLGA not only increased the glass transition temperature, thermal decomposition onset point, and compressive modulus of the composite but also enhanced the surface wettability, which supported cell survival and increased bone tissue regeneration. Xia et al. [61] prepared the 3D-printed PLGA/HA/MgO (PHM) composite porous scaffolds by incorporating nano-hydroxyapatite (HA) and magnesium oxide (MgO) into a PLGA matrix. The experiments showed that, after adding inorganic components (HA and MgO), the composite scaffold could maintain its original pore structure throughout the degradation process with no significant change in surface morphology and showing a stable and slow degradation rate. What is more, the prepared PHM scaffold had good hydrophilic and mechanical properties similar to natural bone and a strong affinity for cell adhesion and proliferation, which can reduce inflammatory reactions and promote the formation of new bone. The porous scaffolds show a good application prospect in the field of bone repair. In another study [62], quaternary ammonium chitosan (HACC) was grafted onto a 3D printing scaffold composed of PLGA and hydroxyapatite to design a bone engineering scaffold with antibacterial and bone conduction properties (Figure 2B). The experiment proved that the combination of PLGA and HA enhanced the mechanical properties and bone conductivity of the scaffold. HACC reduced bacterial adhesion and biofilm formation in vitro and in vivo, and the scaffold generally showed good neovascularization and tissue integration.
2.2.3. Polycaprolactone (PCL)
Polycaprolactone is a class of biodegradable aliphatic polyesters with insufficient mechanical strength. The incorporation of nanoparticles into the PCL matrix can improve the inherent properties of PCL. Jakus et al. [65] have compounded 90 wt% hydroxyapatite and 10 wt% polycaprolactones to form a liquid ink, which was printed by rapid 3D extrusion at room temperature into a synthetic bone regeneration biomaterial (HB). The obtained HB showed high elastic mechanical properties (~32 to 67% strain to failure, ~4 to 11 MPa elastic modulus) and absorbent (50% material porosity. Animal experiments proved that HB could be integrated rapidly with surrounding tissues to support new bone growth without the addition of biological factors. Yeo et al. [66] have proposed a PCL hierarchical structure. First, the PCL pillar was printed using a melt pressure of 3d; then micro/nanofibers were deposited on the surface of the PCL scaffold using an improved electrospinning method. Finally, cell-loaded bioinks (C2C12 myoblasts, polyethylene oxide, and alginate) were printed to the surface of the pillar. The experiments showed that the vertical PCL scaffolds provided excellent mechanical properties. The effective release and uniform distribution of cells in the scaffold were achieved by cell printing, and myoblast extension was induced by aligned micro/nanofibers. This printing method is expected to be used for muscle tissue regeneration. Ji et al. [67] have printed composite scaffolds by mixing nano-hydroxyapatite(nHA) into polycaprolactone and then implanted the hydroxypropyl chitin hydrogel embedded with mesenchymal stem cells to evaluate the osteogenic activity and mechanical properties (Figure 2C). The Young’s modulus of the printed PCL/nHA structure under compression was 2.34 ± 0.10 MPa and 2.33 ± 0.27 MPa, which was much higher than HPCH hydrogel (1.0 kPa). The co-culture experiment showed that the hybrid scaffold promoted macrophages to secrete growth factors, thereby promoting angiogenesis and osteogenesis.
2.2.4. Polyurethane (PU)
The polyurethanes referred to here are thermoplastic linear polymers with biodegradability, high mechanical strength, softness, and high elasticity that can be processed into three-dimensional (3D) scaffolds by various fabrication techniques [72,73]. Chen et al. [64] blended thermoplastic polyurethane (TPU) with polylactic acid and added graphene oxide (GO) for fused deposition molding to achieve 3D printing of elastic TPU/PLA/GO nanocomposites (Figure 2D). They found that the addition of GO significantly improved the mechanical properties of the polymer matrix with a compressive modulus of 167% and a tensile modulus of 75.5%. The cell culture results showed that the 3D-printed scaffolds had good cell viability, which was conducive to cell proliferation, and had good potential as a tissue engineering scaffold. Chen et al. [63] prepared a novel high-viscosity, direct-printable polyurethane composite ink containing nanocellulose (CNF) and fused deposition 3D printing at low temperatures to fabricate various shapes and complex degree brackets. TEM images declared that CNFs were connected to multiple PU nanoparticles to form a “string” structure, and the continuous proliferation of fibroblasts was also proved in the scaffold by in vivo experiments. Hung et al. [68] printed a scaffold at low temperatures using polyurethane (PU) elastic nanoparticles, hyaluronic acid, and bioactive components, which were compliant and bioactive. When the scaffold was implanted into a rabbit cartilage defect, the release of bioactive components promoted the self-aggregation of mesenchymal stem cells (MSCs), which induced the differentiation of MSCs into chondrocytes and produced a cartilage repair matrix.
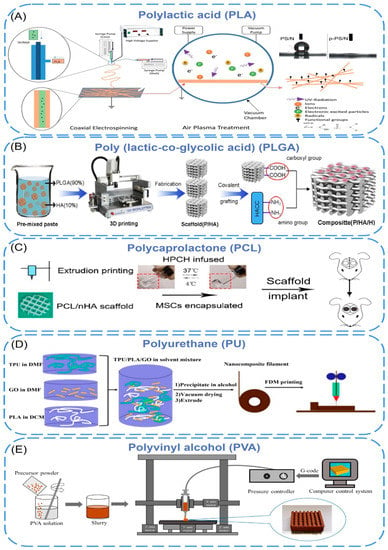
Figure 2. (A) Schematic illustration of the electrospinning and air plasma treatment towards the formation of p-PS/N scaffolds. (B) Schematic illustration for the synthesis mechanism of P/HA/H composite scaffolds. (C) The schema of PCL hybrid scaffold fabrication and in vivo implant. (D) TPU/PLA/GO nanocomposites filament preparation and FDM printing process. (E) Schematic diagram of 3DGP for preparing the porous scaffolds.
2.2.5. Polyvinyl Alcohol (PVA)
Polyvinyl alcohol (PVA) is a synthetic polymer with good biocompatibility, and its viscoelastic properties are comparable to those of articular cartilage. With the addition of nano-additives, PVA scaffolds are an ideal matrix for tissue repair and regeneration [74]. H. et al. [69] prepared porous scaffolds by chemical coprecipitation and 3D gel printing technology. The printing paste based on polyvinyl alcohol and MgFe2O4 nanoparticles has a compressive strength between 1.8–12.51 mpa, which is suitable for human cancellous bone. A preliminary biological experiment showed that MC3T3-E1 cells had good adhesion and proliferation on the MgFe2O4 scaffolds surface, and MgFe2O4 scaffolds can promote ALP activation and calcium deposition, improving osteogenic ability. All this shows that MgFe2O4 magnetic scaffolds are suitable for cancellous bone repair (Figure 2E). Topsakal et al. [70] developed PVA-based 3D-printed composites as versatile biomaterials for orthopedic applications, where gold nanoparticles (AuNP) and ampicillin (AMP) were used for reinforcement. It was shown that the 3D-printed composite scaffolds based on PVA/AuNP/AMP had good biocompatibility, osteoinductivity, and antibacterial properties. Song et al. [71] fabricated 3D-printed scaffolds composed of nano-biphasic calcium phosphate (BCP), polyvinyl alcohol, and platelet-rich fibrin (PRF) at low temperatures with specific shapes and operable internal structures. The scaffold showed good bioactivity and biocompatibility both in vitro and in vivo. What is more, it also proved that PRF has the potential to enhance the repair of segmental bone defects in vivo.
2.3. Polymer Derivatives
Natural polymers are acquired from natural materials and provide an assurance of natural restructure, biomimetic nature, and bioactivity [75]. Natural polymer derivatives refer to more complex products which derived from the substitution of atoms or atomic groups of natural polymers. Generally, the function of a nano-filler is to solve a specific problem.
2.3.1. Chitosan Derivatives
Chitosan has a changeable structure and abundant functional groups. The presence of a large number of amino groups allows chitosan to chemically react with anionic systems to change physicochemical properties. The use of functionalized chitosan to improve the self-healing properties of materials is the most commonly reported. Liu et al. [76] developed a chitosan self-healing hydrogel using modular 3D printing and secondary post-printing crosslinking, in which chitosan was functionalized with phenol (Chi-Ph) and then terminated with telespiral polyethylene glycol (DF-PEG) of benzaldehyde to form injectable hydrogel (CPDP) as printing ink (Figure 3A). The dynamic imine bond between amine on Chi Ph and benzaldehyde on DF-PEG, as well as the irreversible phenol bond between Chi-Ph chains, enable the hydrogel to have a faster gelling rate, higher modulus, better long-term stability, and self-healing ability. These properties may expand the biomedical applications of chitosan self-healing hydrogels. Ko et al. [77] selected ethylene glycol chitosan (GC) and oxidized hyaluronic acid (OHA) as basic materials, formed self-healing iron gel (GC/OHA/SPION) in the presence of superparamagnetic iron oxide nanoparticles (SPION), and then produced 3D structures through extrusion-based printing (Figure 3B). The reversible imine bond between OHA and GC is the key to inducing gel self-healing. Complete self-healing of GC/OHA/SPION ferrogel was observed at SPION > 5 wt%, and this autonomous healing was repeated several times, indicating that the gels were self-healing. Besides, by testing the viability of ATDC5 cells in the presence of polymer solutions or hydrogels, it was found that GC, HA, OHA, and GC/OHA/SPION gels did not show significant toxicity. It may have the potential as a magnetically actuated system in medicine, as it can stimulate and regulate cell differentiation in the presence of the magnetic field. Koosha et al. [78] prepared glyoxalylated chitosan/polyvinyl alcohol (PVA) hydrogel nanofibers by electrospinning in situ and introduced halloysite nanotubes (HNT) as a reinforcing agent, which increased the swelling degree from 272% to about 400%. Biocompatibility studies have shown that the presence of HNT increased the cells attached to the surface of the nanofibers, resulting in higher biocompatibility.
2.3.2. Cellulose Derivatives
To improve the printability (rheological properties) and the formability of the nano-cellulose ink, nano-based auxiliary materials have been added to cellulose derivatives. The principle is to improve performance through particle-polymer interface interactions (electrostatic, van der Waals force, hydrogen bonds, covalent bonds, etc.) and energy dissipation. Xu et al. [38] demonstrated 3D printing and UV crosslinking of cellulose nanofibril (CNF)-based inks containing methacrylate derivatives. The CNF/gelatin methacrylate (GelMA) and CNF/galactomannan methacrylate systems were cross-linked with photoinitiators. By tuning the compositional ratio between CNF and GelMA, the compressive of Young’s modulus and local surface stiffness could be well tuned. The developed ink formulations are noncytotoxic and cytocompatible. Furthermore, the ink formula with CNF incorporated of GelMA, particularly with CNF/GelMA ratios of 2:1 and 5:1, promoted the proliferative activity of 3T3 fibroblasts in comparison with the plain CNF hydrogel. Kuzmenko et al. [79] proposed a conductive nanocellulose-based ink for 3D piezoelectric microvalve printing of neural scaffolds. The ink is composed of highly charged carboxymethyl nanocellulose (CNF) and water-based single-walled carbon nanomaterials (CNT) dispersions. The negatively charged deprotonated carboxyl groups on the fiber surface not only provided electrostatic repulsion between CNFs, but also CNTs could be better dispersed in water (Figure 3C). Cell culture studies showed that neural cells were preferred to attach, proliferate, and differentiate on CNF/CNT scaffolds. Cernencu et al. [80] designed a novel bio-based ink formulation suitable for 3D printing using pectin and carboxylated cellulose nanofibers. Rheological experiments demonstrated that the viscosity of the gel was determined by the nanofibers. The addition of pectin intensifies by increasing the viscosity drop, improving the printability of the ink formulation. The 3D-printed scaffolds based on pure CNF filaments presented a partial instability of the filaments that generate circular pores. The porous objects prepared with the new formulation showed a uniform shape and size of square-like pores, indicating that the bracket is uniform and stable after printing.
2.3.3. Gelatin Derivatives
Gelatin, as a hydrophilic biomacromolecule, is denatured collagen with better processing properties than collagen, but gelatin does not have enough mechanical stability to support porous scaffolds. Gao et al. [81] used macromonomer gelatin methacryloyl chemically crosslinked gelatin (GelMA) to blend with nano-hydroxyapatite and combined with the sol-gel transformation (physical gel) of gelatin derivative aqueous solution to extrude a new physical hydrogel by 3D printing (Figure 3D). Itshowed that the porous bilayer scaffold composed of the hydrogel can induce osteochondral regeneration in vivo after implantation in mammalian joints. Pu et al. [82] printed GelMA/HAp scaffolds by direct ink writing using gelatin methacrylate anhydride (GelMA) with a high solid content of 82.5%, which was mixed with nano-hydroxyapatite (HAp) particles and anchored with functionalized polyphenol hydrogels. The scaffold and hydrogel were organically integrated into a biomimetic bone implant, which exhibited better water retention and mechanical stability than a single 3D-printed scaffold. It can promote the migration, proliferation, and osteogenic differentiation of bone marrow stem cells. Boere et al. [83] used poly(hydroxymethylglycolide-co-e-caprolactone)/poly(e-caprolactone)/PCL to functionalize thermoplastic polymer blends (pHMGCL/PCL) and grafted it onto gelatin methacrylamide (GelMA) hydrogel by photopolymerization (Figure 3E). Three-dimensional printed PHMGC/PCL and pMHMGCL/PCL scaffolds were used to enhance GelMA constructs and tested in a model of local articular cartilage defects. It was found that chondrocytes embedded in the constructs could form cartilage-specific matrices in vitro and in vivo. The results demonstrated that the embedded chondrocytes displayed significant cartilage-specific matrix deposition in these constructions.
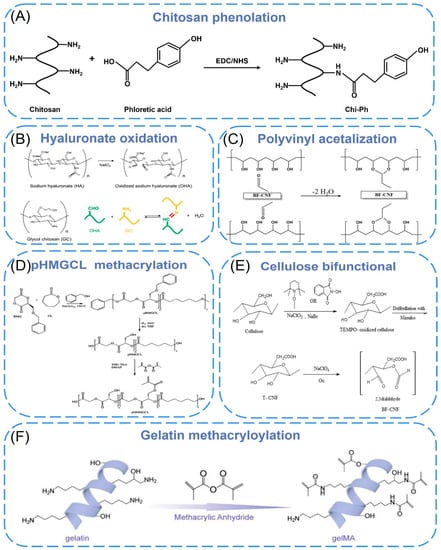
Figure 3. (A) Schematic illustration for conjugation of phloretic acid to chitosan using EDC/NHS chemistry. (B) Chemical structure of OHA and GC, and Schiff base formation between OHA and GC. (C) Chemical structure of carboxymethyl cellulose. (D) Functionalized pHMGCL/PCL with methacrylate and covalently grafted to gelMA hydrogel. (E) Synthesis route for nanofibers with aldehyde and carboxylic acid groups. (F) The reaction diagram of the synthesis of gelMA.
This entry is adapted from the peer-reviewed paper 10.3390/biomimetics8010094
This entry is offline, you can click here to edit this entry!
