1. Introduction
Dated back to 1888, more than 130 years ago, Mr. A.N.S. saw the beautiful appearance of snowflakes glowing in the dark night when exposed to sunlight, raising a few questions about how these crystals are formed and why they look so fascinating
[1]. It has been a remarkably long history since people started to dig into one of the most ubiquitous phenomena encountered in nature and human activities, i.e., how water vapor gets frozen and the consecutive processes such as the formation of frost, atmosphere icing (snow/hail/rime/frozen rain), accretion of frost in HVAC (heating, ventilating, and air conditioning) systems, and snow/ice making in sports and entertainment business
[2][3]. Condensation frosting is one of the most pervasive types of ice that we encounter in many application backgrounds. The structural integrity and mechanical reliability of infrastructures and facilities, such as power transmission systems
[4][5], aircraft, and wind turbines
[6][7], can be compromised to a considerably large extent due to frost accretion whenever exposed to humid and supercooled environments. For heat transfer equipment such as refrigerators and heat pumps, both their heat transfer efficacy and capacity will be tremendously reduced once the interfaces are covered by thick porous frost layers
[8][9][10][11]. Billions of economic losses or even the cost of human lives due to frost/ice accretion have occurred multiple times, such as the cold weather strike across southern China in 2018 and the Texas power crisis in 2021.
2. Mechanism of Condensation Frosting: 5 Consecutive Stages
Condensation frosting is a complex heat and mass transfer process composed of two steps of phase change: condensation when ambient vapor becomes oversaturated and nucleates on the liquid–substrate interface, and icing when condensed drops freeze and subsequently frost dendrites grow atop frozen drops. Although the occurrence of desublimation, i.e., vapor directly turns to ice on sufficiently hydrophilic surfaces and at cold enough temperature (contact angle smaller than 50°, substrate temperature lower than −35 °C)
[12], is also widespread, this text solely focus on the more general cases of condensation frosting. As shown in
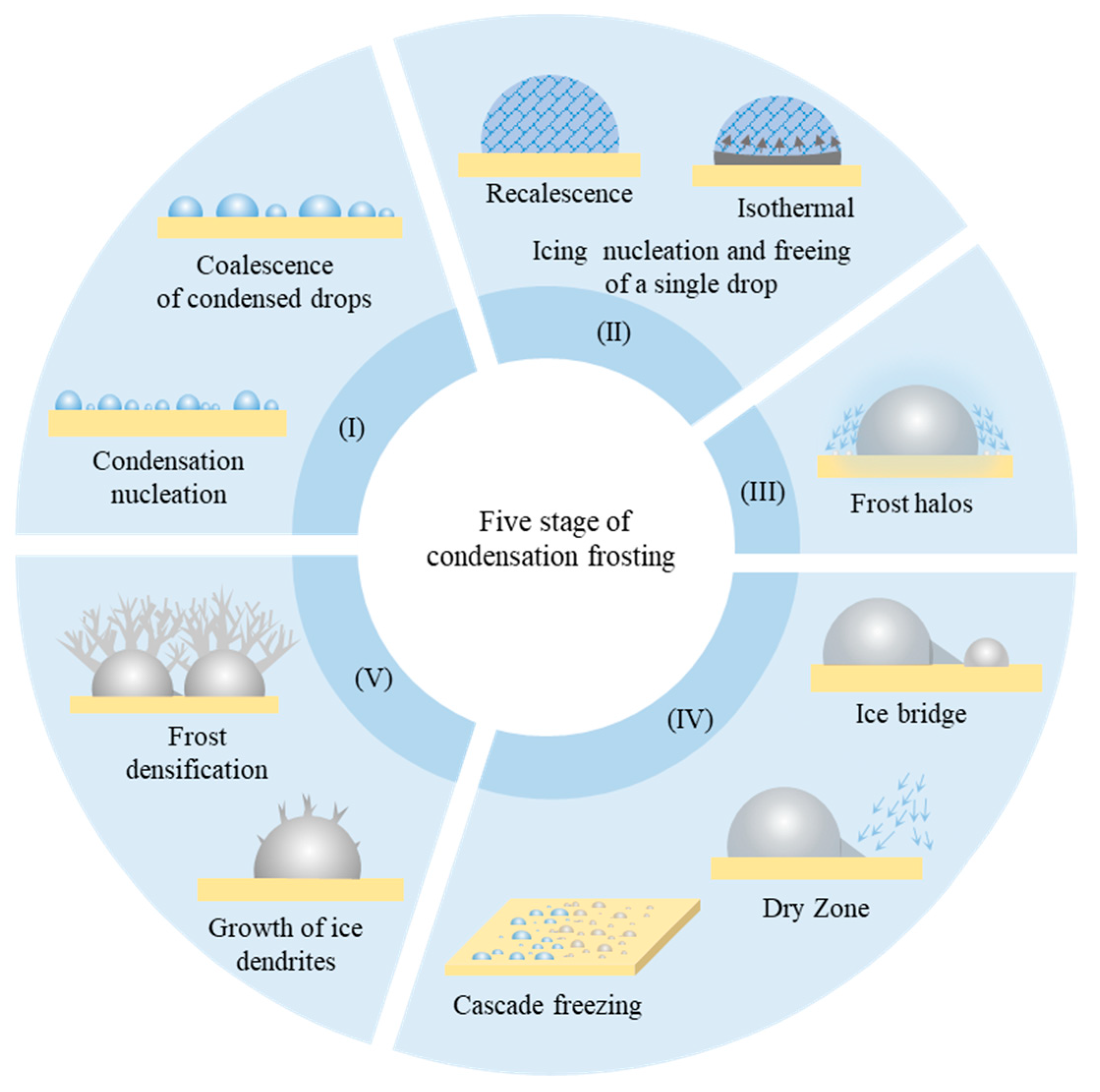
Figure 1, researchers characterize the whole physical process chronologically into five sequential stages: (I) nucleation, growth, and coalescence of condensed drops; (II) icing in one of the condensed drops; (III) evaporation-induced condensation/frost halos; (IV) freezing propagation via ice bridging; and (V) growth of ice dendrites and frost densification.
Figure 1. Five physical stages of condensation frosting.
2.1. Condensation Nucleation and Interdrop Coalescences
Condensation refers to the formation of nanoscale liquid condensate once its vapor is oversaturated, or in other words, the solid surface is supercooled for the heterogeneous mode. For water vapor specifically, a certain degree of supercooling beneath the dew point is necessary to overcome the heterogeneous energy barrier ΔGHetero required to create a stable nucleating embryo (with a minimal radius r≥rcri, rcri being the critical radius for condensation nucleation).
As the incipient stage of the whole physical process, the location and density of nucleation sites dominate the distribution of condensate, which in turn plays a decisive role in successive frosting stages. Both surface chemistry and topological features can be tailored to modulate condensation dynamics. Boreyko et al. used engineered surfaces carrying solely chemical patterns to spatially control the nucleation sites
[13]. Arrays of hydrophilic dots were patterned again on a hydrophobic background, engendering a typical wettability contrast. The energy barrier for condensation nucleation in hydrophilic regions is significantly smaller than that of hydrophobic regions, providing preferential nucleation sites where stable condensed drops emerge and start to grow bigger in size. Zhao et al. fabricated silicon-based topological patterns to control the size and distribution of condensed drops
[14][15]. Micropillar patterned surfaces with varying diameters and pitches were fabricated using standard photolithography. The prepared surfaces are macroscopically superhydrophobic if placing a millimeter-sized water drop atop. As the surface is chemically homogeneous, condensation nucleation occurs uniformly on the substrate bases, as well as the pillar tops and sidewalls. However, condensed drops atop pillars are subjected to much larger feeding flux, and thus can grow faster. Accordingly, we can observe that pillar tops collect most of the condensate in the late stage of condensation. Recently, engineered surfaces with both chemical and topological patterns were also exploited. Hou et al. designed and fabricated a surface that carries a biphilic topography with patterned high-contrast wettability
[16]. The prepared surface is composed of arrays of hydrophilic micropillars and superhydrophobic nanograss. The condensation dynamics on this biphilic surface were captured via environmental scanning electron microscopy (ESEM). Condensate drops preferentially nucleate atop hydrophilic pillar tops, and superhydrophobic nanograss assists the drops remaining suspended.
As condensation proceeds and more vapor is fed continuously, condensate drops grow larger in size and merge with neighboring drops, which is usually termed interdrop coalescence. Accordingly, researchers believe the transition from dropwise condensation to filmwise condensation is inevitable without applying external forces. Boreyko and Chen found that continuous dropwise condensation is possible on properly designed superhydrophobic surfaces
[17]. Condensate drops can jump spontaneously upon the coalescence with neighboring drops due to the release of excess surface energy. A surprising out-of-plane jumping motion with a speed up to 1 m/s can result from an in-plane coalescence of two micrometer-sized drops. Later, they further implant this mechanism into phase-thermal thermal diodes to promote heat transfer efficiency with a strong directional preference
[18]. Nenad et al. discovered that jumping drops carry a net positive charge, yielding a self-repelling behavior in the mid-fight
[19]. They used externally applied electric fields to modulate the kinetics of jumping drops and, more specifically, to increase the jumping frequency from the substrate to promote condensation heat transfer.
2.2. Icing Nucleation and Freezing of a Single Drop
As deduced from Fletcher’s classical nucleation theory
[20], icing nucleation occurs inside condensed drops at energetically preferential sites, analogous to that of condensation nucleation. For icing nucleation in supercooled sessile drops, there has been a long-lasting debate about whether the triple line is the spatial preferential nucleation site. Gurganus et al. developed a direct experimental approach to study the spatial distribution of nucleation sites for a sessile supercooled drop
[21][22]. Two high-speed cameras from both the top view and side view allow pinpointing precisely the location and growth dynamics of the ice embryo. The hexagonal ice crystal initiates from an arbitrary location at the liquid–solid interface, showing that icing nucleation has no preference for the triple line.
Different from the scenario of the freezing of an impinging drop, condensed drops are sessile and supercooled before icing nucleation initiates. Despite that nucleation sites also locate at the liquid–solid interface, the whole freezing dynamics can be very different. Jung et al. found that the freezing process of a sessile supercooled drop proceeds in two consecutive stages, termed the recalescence stage and the isothermal stage, respectively
[23]. In the first recalescence stage, a rapid partial freezing occurs, and the whole drop space gradually becomes cloudy as freezing proceeds. Most of the released latent heat is absorbed by the remaining water to raise its temperature, while a small portion is dissipated to the surroundings. In the second isothermal stage, the remaining water gradually transits into a solid, filling the crystalline gaps formed during the recalescence stage. The released latent heat in this stage is mostly transferred to the substrate base. In general cases, the isothermal stage proceeds near 3 orders of magnitude slower than the recalescence stage.
An intriguing fact about water is it expands upon freezing, and thus the shape of a drop evolves as freezing proceeds. Marín et al. carried out a set of experiments to study the shape of frozen drops, and proposed a quantitative description
[24]. For both 3D drops and 2D drops (as confined in a Hele–Shaw geometry), the top portion of a frozen drop is a conical tip, which is independent of contact angle and substrate supercooling temperature.
2.3. Freezing Halo
The formation of frost halos initiates at the onset of icing nucleation, and thus chronologically is partially overlapping with the drop icing stage. Before the recent work by Jung et al.
[25], people used to formulate frost halos as the result of the spontaneous ejection of micrometer-sized drops when a large drop starts to freeze. An appreciable amount of vapor is released from the air–liquid interface when the main drop freezes and heats up the remaining liquid. This vapor becomes locally supersaturated and deposits surrounding the main drop, engendering a halo consisting of micrometer-sized condensate drops. These condensate drops inside the halo are either frozen into ice drops forming so-called frost halos, or evaporated off if too remote from the main drop. Vapor from the evaporated remote condensate drops is transferred via diffusion and redeposited on the neighboring frost halo. Furthermore, they compared the halo formation on surfaces with varying thermal conductivity. On low thermal conductivity materials, the freezing time of the main drop is longer, and thus more evaporated vapor is released. Condensed drops can grow, coalesce, and freeze, yielding a large freezing halo. On high thermal conductivity materials, the main drop freezes drastically faster, cutting off the source of feeding vapor flux. More condensed microdrops are evaporated off before freezing, yielding a small freezing halo.
Note that when one single drop among condensed drops gets frozen, ice covers not only the frozen drop itself, but also a radial area to an appreciable extent, explaining why frost coverage is significantly larger than condensate coverage for the same thermal physical circumstances. Such frost halos were also reported recently by Zhao et al. when they deposited a warm drop onto supercooled surfaces
[26]. Even for the case of a surface temperature higher than the freezing point, a similar halo-like configuration was observed, whereas frost halos transit into condensate halos instead
[27].
2.4. Freezing Propagation via Ice Bridging, Dry Zones
As late as 2010, people started to notice that icing in condensation frosting follows a pattern instead of a collection of individual incidences
[28][29]. Interdrop dynamics, referred to as freezing wave or ice bridging, are exploited to describe the process of how an ice dendrite initiated from a frozen condensate extends to contact with neighboring liquid drops and freezes them. Accordingly, icing nucleation only occurs in energetically preferential sites such as topological cavities and chemical defects, and then limited frozen condensates set off a chain reaction spreading over whole supercooled surfaces.
Ice bridging is a multistep phase-change phenomenon coupled with spatial and temporal variations of heat and mass transfer, and thus a quantitative physical description that matches experimental observations is impractical. Boreyko and Collier employed a simplified two-dimensional physical model to describe how a typical ice bridge is constructed
[30]. Once a condensate drop is frozen, a vapor concentration gradient is built between its ice interface and neighboring liquid interface. The neighboring liquid drop evaporates, and the evaporated vapor is deposited onto the frozen drop to construct the ice bridge. As the evaporation and the deposition proceeds, the ice dendrite expands towards the neighboring drop, which meanwhile shrinks in size due to the evaporative loss of mass. A scaling analysis that correlates the dendrite growth rate and the evaporative mass loss can be applied to evaluate the time scale to construct the ice bridge. Note that a frozen drop has multiple neighboring liquid drops and vice versa, and a sequence presents when frost spreads across a group of condensate drops. Guadarrama-Cetina et al. experimentally investigated the routine of frost spreading when 2D ice bridges percolate through a network of condensate drops
[31]. A growing dendrite points to the neighboring liquid drop and a successful ice bridge is constructed if the liquid drop is sufficiently close and large enough. A failed percolation, or a partially constructed ice bridge, occurs when the liquid drop is evaporated off before any dendrite reaches. Accordingly, a considerable number of liquid drops that are too small in size and too remote vanish during frost spreading, leaving depleted dry zones around the frozen drops and ice bridges. Hauer et al. studied the pattern formation in frost spreading using laser-induced fluorescence microscopy
[32]. Varying modes of frost spreading on microstructured surfaces were revealed by setting the degree of supercooling and the time of condensation.
The scenario becomes drastically different when condensate drops are suspended. Zhao et al. developed an analytical model to describe quantitatively the time required to construct a 3D ice bridge
[14][33]. A series of silicon-based micropillar patterned surfaces were employed to control precisely the size and distribution of condensate drops. Icing nucleation occurs at substrate edges which carry topological defects, allowing us to further control the direction of ice bridging. The geometry of a typical 3D ice bridge is considered to be of a circular cone. They also assumed that vapor transfer is governed by one-dimensional diffusion. The time required to construct one single ice bridge can be computed by balancing the mass transfer rate and the total bridge mass, based on which a spatial average frost spreading velocity can be obtained.
2.5. Dendrite Growth Atop Frozen Drop and Densification
As a conical tip presents atop the frozen drop due to the volume expansion upon freezing, this phenomenon engenders an interesting consequence in the succeeding growth of ice dendrites when dealing with condensation frosting. The singular tip is exposed to a significantly large feeding flux driven by the vapor concentration gradient, making it a preferential site for vapor deposition (physically can be termed desublimation
[34]). Accordingly, ice dendrites grow like a tree, as reported by Enriquez et al.
[35]. Yu et al. measured quantitatively the growth dynamics of ice dendrites atop frozen drops at varying supercooling temperatures
[36]. In general cases of ordinary-low temperatures, needle dendrites grow into columnar shapes, and their tips melt when exposed to thermal fluctuations. Their upper interfaces gradually change into lumps, and overall dendrites grow into larger sizes. At relatively lower cryogenic temperatures, the growth of ice dendrites is much slower. Vapor is frozen into ice clusters and deposited atop the drop instead. As frosting proceeds, thicker ice clusters cover the drop and inhibit the growth of dendrites. Huang et al. further evaluated the effect of surface orientations with respect to gravity on the growth rate of ice dendrites as vapor transfer occurs via natural convection
[37]. They found both the average vapor deposition rate and its spatial variation change profoundly by altering surface orientation. For a horizontally placed surface, detectable dendrites grow all over the frozen drop, with an appreciable larger growth rate at the singular tip, named the “global peak”. Later, vapor deposition near the global peak is suppressed to some extent, yielding two secondary “local peaks”. For a vertically placed surface, the frozen drop is unsymmetrical due to the effect of gravity. Ice embryos emerge only at the upper portion of the frozen drop, and eventually, only the upper portion is covered with a collection of ice dendrites, leaving a smooth ice-air interface for the bottom portion of the frozen drop. The results show that natural convection can either assist or hinder the vapor diffusion process, and affect the deposition outcomes.
Once the entire surface is covered by a network of frozen drops and ice bridges, dendrite growth atop the frozen phase leads to crossing, reverse melting, and collapses, and thus frost density evolves over time
[38][39][40][41][42]. Different from other incipient condensation frosting stages, frost densification has been studied extensively for decades. Hermes et al. developed a physical model to describe the variation of the frost density over time based on the mass and energy balances within the frost layer
[43]. In such cases, the frost layer is assumed as a porous medium exposed to supersaturated moist air. Song et al. experimentally measured the frost density at varying surface supercooling temperatures
[44]. They found that the reverse melting can profoundly increase the frost density, and thus the obtained frost density increases with the increase of the surface temperature. Wang et al. studied experimentally the effect of surrounding humidity on frost density
[45]. For larger humidity, more water vapor penetrates the frost interface and diffuses into the inside layer in the desublimation form, yielding a larger frost density. Shin et al. studied the effect of surface wettability on frost density
[46]. Their experimental results show hydrophobic surfaces allow the presence of large irregular ice crystals in the earlier stage of frost deposition, and thus result in a low frost density. Thermal physical properties such as thermal conductivity, heat transfer coefficient, and thermal capacity, depend strongly on the frost density. Therefore, in view of anti-frosting technical attempts, proper design to increase frost density is preferred for HVAC devices.
This entry is adapted from the peer-reviewed paper 10.3390/cryst13030493