Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Physiology
As an essential micronutrient with a nearly ubiquitous presence in nature, zinc is needed for all known aspects of life. Based on the quantification of putative zinc protein binding domains, it is estimated that one-tenth of all human proteins require zinc as a structural element or for an enzyme active site. The structural, catalytic, and regulatory roles of zinc provide the foundation for a broad array of basic cellular functions. Consequently, zinc deficiency affects numerous critical functions, including metabolic, immune, and neurological processes. With zinc nutriture underlying a public health burden of communicable and non-communicable diseases, human zinc deficiency is estimated to be the most prevalent nutrient deficiency worldwide. The etiology of human zinc deficiency has historically been attributed to diets with low zinc bioavailability, e.g., the proportion of dietary zinc available for zinc-dependent functions, with primary attention to diets low in zinc and high in phytic acid.
- zinc
- bioavailability
- absorption
- phytate
- protein
- calcium
1. Unique Chemistry of Zinc
Zinc is a redox-inactive divalent transition metal. Its biological activity is determined primarily through its ability to serve as a Lewis acid for catalysis or as a component of protein structures and superstructures and its coordination with amino acid side chains for regulating its various chemical and structural roles [14,15,16]. The bonds between zinc and the side chains of zinc-binding amino acids and between zinc and water thus determine the nature of its biological function.
The binding of zinc ions is unique compared to other transition metals. The coordination of aqueous zinc may include four, five, or six water molecules [14]. In its catalytic roles, zinc is coordinated between three amino acids (including one or more histidine residues) and one or more water molecules [17]. As with aqueous zinc, the zinc in active catalytic sites on proteins may take on a coordination number of four, five, or six. In contrast, structural zinc is frequently coordinated between four amino acids, including two or more cysteine residues. Steric repulsion by cysteine sulfur prevents zinc from forming bonds with water. This exclusion of water molecules maintains the neutrality of zinc.
Following the Irving-Williams series, divalent metal binding sites on metalloproteins have the highest preference, or affinity, for copper and zinc and the lowest preference for magnesium and calcium [18]. Free zinc readily displaces other divalent metals from their binding sites [19]. To prevent cytotoxic effects due to the mis-metalation of proteins, free metals are maintained at a concentration inversely proportional to their respective binding preference. Thus, cytosolic free zinc concentration is maintained in the picomolar range [20], more than three orders of magnitude lower than free magnesium or calcium, the two divalent metals with the lowest protein binding preference [19,21].
The natural reactivity of zinc in the presence of water, its ability to readily displace other divalent metals from their protein binding sites, and its broad functional importance as a catalytic and protein structural element require tightly regulated movement of zinc from its intestinal absorption through to its delivery to systemic cells for its numerous catalytic, structural, and regulatory roles [22]. To illustrate the extremity of this regulation of free zinc, consider the number of free zinc atoms indicated by picomolar concentration in the cytosol of a typical cell. Assuming that the cytoplasm makes up about half of the cell volume, and the cytosol makes up 61% of the cytoplasmic volume [23], about 30% of the cell volume is cytosol. Given a cell volume of 200 fL, and cytosolic free zinc ranging from 60 to 270 pM [24], the cytosol of a single cell would only contain between two and ten free zinc atoms.
Similar to copper, affinity gradients facilitate the controlled transfer of zinc [25]. As zinc moves through biological systems, it is transferred from one ligand to another, a process that is more rapid between ligands with similar binding affinities [26,27]. This controlled transfer of zinc is primarily mediated by a complex network of zinc carriers and transporters. However, unlike copper, zinc does not have a strong tendency towards a particular bond geometry [14], allowing more flexibility for zinc-binding sites to serve dual roles as zinc donors simply due to their relative zinc binding preference and proximity to an acceptor zinc-binding site. With this background regarding the unique chemistry of zinc and the nature of its movement in biological systems, the following section discusses the known molecular mechanisms underlying zinc bioavailability.
2. Zinc Bioavailability
Nutrient bioavailability is defined as the proportion of a nutrient in the diet utilized in the body [9]. Since this utilization is often difficult to quantify in a way that is traceable to diet, the concept of bioavailability is divided into three phases: availability for absorption in the intestinal lumen; absorption, retention, and distribution in the body; and tissue and cellular utilization [9]. While nutrient availability or absorbability may serve as estimates of bioavailability, zinc bioavailability is most correctly defined as the proportion of zinc in the diet that is ultimately utilized in zinc-dependent functions (Figure 1).
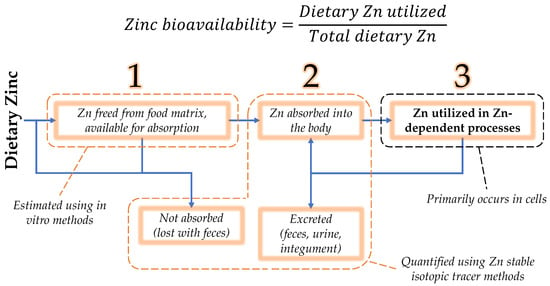
Figure 1. Zinc bioavailability is the dietary zinc that is utilized in zinc-dependent processes, expressed as a fraction or percent of the total dietary zinc. Towards its estimation, bioavailability may be separated into three phases: (1) luminal availability, (2) absorption/retention/distribution in the body, and (3) utilization [9]. Zinc that is absorbed may be retained for utilization, secreted endogenously, as a component of pancreatic juice, or directly by intestinal cells. Secreted zinc may be reabsorbed or excreted in the feces.
Bioavailability describes a dietary quality with a typical emphasis on the components that are the most readily measured, luminal availability, and absorption. However, this emphasis encourages the assumption that once zinc is absorbed, its chance of utilization is not further modified by dietary or physiological factors. In an article on nutrient bioavailability in humans and experimental animals, JS Godber cautioned that bioavailability should account for “intrinsic and extrinsic factors that may influence the ultimate utilization” [28].
A related term, drug bioavailability, is defined as the proportion of dose that reaches the site of action in its active form [29]. A major distinction between nutrient and drug bioavailability is the presence of food. Zinc is naturally a component of food, although dietary zinc may be provided artificially as a fortificant or as orally administered supplement pills. The latter may be taken fasted or with food. Other therapeutic forms, such as those used for topical administration or injection, do not interact directly with the gastrointestinal tract or digestive processes that determine nutrient bioavailability. Thus, either or both definitions could apply to zinc, primarily depending on whether the purpose is nutritional or therapeutic.
2.1. Zinc Luminal Availability and Absorption
Upon ingestion of a meal, digestive processes begin to free the dietary zinc from the food matrix, making it available for absorption. The acidic gastric environment hydrolyzes dietary zinc and, after passing the pyloric sphincter into the duodenum, pancreatic juices containing bicarbonate and digestive enzymes mix with the digestate, neutralizing the gastric acids and begin hydrolyzing dietary protein, and thereby releasing protein-bound dietary zinc. Pancreatic juices contain a substantial amount of zinc. Several milligrams of zinc are secreted daily in pancreatic juices [30,31], whereas only about 2.5 to 3.5 milligrams of zinc per day are absorbed from food to meet physiological requirements [32]. Plasma zinc kinetics following oral zinc administration are consistent with enterohepatic recirculation, whereby absorbed zinc is secreted with pancreatic juices and reabsorbed [33].
Dietary zinc is thus mixed with digestive juices and endogenously secreted zinc, components of food that have been made soluble through digestive processes and continue to be broken down, and those that remain insoluble. Reducing this to a molecular perspective of zinc before its absorption, zinc in the digestive matrix is bound to water molecules and numerous zinc-binding ligands of varying affinities.
In the context of digesting foods, soluble zinc-binding ligands, such as peptides and amino acids, help to maintain the solubility of zinc and thus promote its access to zinc transporters. When these ligands are less soluble (e.g., undigested protein or phytate complexed with calcium), they may be capable of precipitating zinc, thus preventing its contact with the active sites of zinc transport proteins. It is primarily the interaction of dietary zinc with water, zinc-binding ligands, and the zinc-binding sites on zinc transport proteins that determine the availability of zinc for absorption.
In the context of zinc-fortified foods or supplements, additional complications may be present. For example, when a zinc supplement or tracer is administered in the post-absorptive (fasted) state, the fractional absorption and appearance in plasma are much greater than normally observed with food [34,35]. This suggests that foods and/or digestive processes reduce zinc bioavailability. However, it is also known that zinc is rapidly drawn from plasma, predominantly directed to the liver, with the intake of food [36].
Furthermore, direct comparisons of any marker of cellular zinc utilization, beyond plasma zinc concentration, when zinc is taken fasted vs. with food are startlingly few. The effects on plasma zinc concentration and on a zinc-dependent cellular process lack congruence [37]. Although zinc taken in the fasted state daily for two weeks has a greater effect on plasma zinc concentration, the effect on zinc-dependent essential fatty acid desaturation is more pronounced when the zinc is taken with food.
In addition, the forms of zinc used for these purposes may have unique metabolic qualities atypical of zinc as a natural component of food. Consider zinc oxide, commonly used as a fortificant and in zinc supplements. Zinc oxide has low solubility and only releases zinc through a reaction that releases reactive oxygen species (ROS) [38]. Zinc given as supplements in the fasted state had a lower fractional absorption when provided as zinc oxide, compared with zinc citrate or zinc gluconate [39]. Some of the participants had undetectable (<1.5%) fractional absorption from zinc oxide, perhaps due to inadequate stomach acidity [40]. Cytotoxicity of ROS released from zinc oxide, even at levels typical of fortification, may also be of concern for gut health [41].
3.2. Zinc Utilization
Absorbed zinc may be directed to utilization in cellular compartments, transported into the urine, or excreted back into the digestive tract. Balance studies indicate a positive linear relationship between zinc absorption and excretion [32,92]. Since there are no body stores for labile zinc readily released in response to low intakes, absorbed zinc that is not utilized in zinc-dependent functions is, in turn, excreted. The more zinc absorbed, the more that is excreted back out of the body. In addition to the total amount of zinc absorbed, little is known of factors that would determine the retention and utilization, versus the excretion, of absorbed zinc.
A review of the proteins that regulate zinc metabolism at the cellular, tissue, and whole-body levels, and their properties, however, may provide important insight into the nature of a zinc utilization axis. Zinc carriers and numerous zinc transporters maintain zinc homeostasis via the regulation of cell and organelle-specific transfer of zinc across membranes and are reviewed by others [22,26,131,132].
2.2.1. Metallothionein
MT is a compact, cysteine-rich protein highly conserved in nature [133]. Mammalian MTs contain 61 to 68 amino acids depending on the isoform, 20 of which are cysteine and are capable of binding up to seven zinc atoms. The MT α domain has four zinc sites with picomolar affinities, while the β domain has three zinc sites with nanomolar affinities [61,134]. The level of intermediate zinc metalation of MT determines its function. As MT takes on zinc, its affinity for additional zinc decreases, suggesting a shift from sequestering zinc (where the zinc would be bound too tightly to exchange with other proteins readily) to zinc donation to enzyme active sites [61].
Due to its range of zinc-binding affinities, MT may moderate the metalation of numerous zinc-dependent apo-enzymes. This is exemplified by carbonic anhydrase 2 (CA2), a zinc-dependent enzyme responsible for the hydration of carbon dioxide to carbonic acid for transport in the blood or towards the production of hydrochloric acid in the stomach. MT donates zinc to the catalytic site of CA2, which has a pKd (affinity) for zinc of 12.1 [64]. Only the last two zinc-binding domains of MT bind zinc with a lower affinity, with pKd of 11.4 and 11.7. Therefore, only when MT carries a total of six or seven zinc atoms may it donate zinc to the active site of apo-CA2 [135]. However, with a zinc influx into the cell, apo-CA2 effectively competes with MT for zinc once MT carries more than three zinc atoms [61], suggesting that transient influxes of zinc are advantageous towards the metalation of CA2.
The role of MT in regulating the cellular response to oxidative stress may be additionally important in determining the direction of zinc toward utilization. MT neutralizes ROS through the oxidation of its cysteine residues [136]. This causes the release of zinc and polymerization of MT [137], which signals a further cellular response to include increased expression of MT [138]. In the presence of ROS, zinc is more readily released from the lower affinity β domain of MT [139].
Thus, an increased demand on MT to respond to ROS would be expected to lead to a concurrent shift in zinc metabolism, reducing zinc transfer to protein active sites, increasing the movement of zinc out of the cytosol, and, with the further expression of apo-MT, increased sequestration of cytosolic zinc to its high-affinity zinc-binding sites. Although it has implications for altered zinc homeostasis and reduced zinc utilization in conditions of oxidative stress, to our knowledge, this has not been explored in metabolic studies of zinc.
2.2.2. Albumin
Albumin is the primary zinc carrier protein in blood plasma, where it mediates zinc transport between tissues. ZnT1, localized to the basolateral membrane of luminal enterocytes, is responsible for the transport of absorbed dietary zinc into the portal blood [131], where albumin collects the zinc for transport to the liver. Without albumin, zinc is not transported out of mucosal cells into circulation [140].
About 80% of the zinc in blood plasma is bound to albumin, which has a single high-affinity zinc-binding site [141]. Most of the remaining zinc in plasma is bound to the protease inhibitor, α-2-macroglobulin [142], which has an affinity for zinc similar to that of albumin [63]. Under normal conditions in the healthy individual, albumin is at a molar ratio with zinc of approximately 40:1, conferring a high capacity of blood plasma to scavenge free zinc [2]. The affinity of albumin for zinc, pKd of 6.4 [62], combined with its molar excess over zinc, effectively keeps free zinc in plasma in the range of 1–3 nM [143].
Albumin is also the carrier protein for free fatty acids (FFA). In the lipolytic release of FFA from circulating chylomicrons, albumin is needed for clearance of FFA from lipoprotein lipase and transport to the receptors mediating cellular FFA uptake [144,145]. In support of this function, the zinc-binding site on albumin serves as the primary FFA binding site [146]. As FFA are released with lipolysis, zinc is released from albumin and thus directed into cellular compartments.
Food intake directs plasma zinc into slow turnover tissue stores that are predominantly localized to the liver [36], consistent with its utilization, an effect mediated by postprandial lipolysis. Due to this postprandial cross-talk between zinc and FFA, food intake determines zinc utilization.
2.2.3. Summary of Zinc Utilization
In exploring the interaction of zinc with its carrier proteins, potential mechanisms underlying an axis of zinc utilization vs. clearance are revealed. Varying affinities of MT zinc sites determine the direction of zinc to the metalation of zinc enzymes, which differs between steady state and conditions of cellular zinc influx. The dual roles of MT in carrying zinc and responding to oxidative stress are interactive in regulating the distribution of cellular zinc. Likewise, the dual roles of albumin in carrying zinc and fatty acids are interactive in regulating postprandial zinc utilization.
This entry is adapted from the peer-reviewed paper 10.3390/ijms24076561
This entry is offline, you can click here to edit this entry!
