1. Introduction
Water is the core resource for every living organism and a significant feedstock for any industry [
1]. The utilization of freshwater generates many effluents or contaminated water that might be directly disposed of and cause significant health and ecological risks. The contaminated wastewater may contain various hazardous materials. In addition, a vast number of inorganic and organic nutrients are released into the environment, reflecting high COD and BOD [
2]. It was reported that 50% of the population faces energy shortages, 70% need food, and 50% need water, with more than 75% wanting to decline CO
2 emission [
3]. One of the most challenging issues is surface water pollution (i.e., hypertrophication), because the globe faces a water shortage. The available reservoir also contaminates wastewater (such as sewage, industrial, non-industrial, medical, and laboratory wastewater). In addition, hypertrophication is caused by the excessive loading of N and P and causes solid waste generation and unwanted emissions into the air [
4]. It also leads to supporting pathogenic microbes that become a significant threat to aquatic life as well as the associated organisms [
5].
Another class of heavy metal pollutant might be directly inhaled, ingested, or in contact with the skin, causing major health issues and escalating the risk of cancer [
6]. The unexplored or partially explored contaminates named emerging pollutants (EP), which were still not appropriately addressed and had severe danger, received attention during the last five years. The scientist focused on EPs [
6,
7,
8,
9,
10], their hazards, and processing to eradicate these contaminants.
When water treatment is a concern, adopting the most effective technique fulfills the purification objective. The conventional methods for addressing the purification process, such as physical, chemical, physio-chemical, membrane technology, and hybrid processes, are commercially used. Still, these processes have some flaws or disadvantages [
11] and shall be evaluated thoroughly before their implementation. The most effective process is the one that has been commercially used on a large scale to alter the water characteristics using chemical dosing, which alters the turbidity, pH, TDS, and TSS. Some other processes include chlorination, coagulation/flocculation, ultraviolet light, and ozonation. However, all these technologies also have economic, recycling, and maintenance issues. The most common technology for water and wastewater treatments applied on a large scale is the biological method that depends on the metabolic activities of microorganisms to decompose and convert pollutants (including toxins) to biomass and associated gases (CH
4, CO, CO
2, N
2, and SO
2) [
12,
13,
14,
15].
The biological approach involves biodegradation with various microorganisms among bacteria, fungi, yeast, and microalgae. This process is not quite simple, involving the metabolic activity to utilize the toxins [
16]. However, biological processes are considered more cost-effective, despite of several restrictions such as a huge area, long retention time, low biodegradation rate, limited design flexibility, and limited ranges of operation conditions [
17].
Biological treatment is typically categorized as the secondary treatment for eliminating mainly biodegradable pollutants that remain after the primary process. Various microalgae genera such as
Scenedesmus,
Chlorella,
Botryococcus,
Phormidium,
Limnospira, and
Chlamydomonas have been reported as remarkable agents for bioremediation.
Scenedesmus,
Chlorella,
Euglena,
Oscillatoria,
Chlamydomonas, and
Ankistrodesmus have shown effective growth and tremendous tolerance against toxins [
17].
Bioremediation is a process that uses the living organism to target the toxins and transform them into safe ends [
18], whereas the biosorption process is the one used to target the toxins via electrostatic attraction on the surface of microalgae [
19]. The microalgae-based technique utilizes both processes, as such gained significant attention for treating diversified wastewater [
20]. Microalgae can reduce hypertrophication by converting it into biomass mass in the presence of sunlight [
21]. Additionally, the microalgae collected from various ponds can be a food source for multiple products [
22]. Another optimized version of biological treatment is the microalgae coupled with any other microbes to speed up the remediation process [
23]. Hence, it can be said that microalgae utilization for wastewater treatment is a big challenge for conventional approaches if the limitation mentioned above is addressed and resolved.
1.1. Classification of Algae
The tiered group of life into empires,
phyla (divisions), groups, etc. precedes the concept of progression [
24]. The grouping triggers dilemmas with classification of monophyly, specifically of the “lower” or less-complex uni- and multicellular types [
25]. In spirit, numerous researchers have endeavored to reclassify the algae, but no effort has been adequate, resulting in numerous distinct categories that are currently available [
26]. The continual retitling and moving of genes from one tier to another do not solve the issue without adequate representation (i.e., phylogenies) [
27]. Currently, it is difficult to create a comprehensive catalog that accurately represents the monophyletic lineages of the
Protista and
Chromista kingdoms. Rather than attempting to create hierarchically-organized catalogs, it may be more beneficial to encourage the development of consortia that can easily be distinguished and added to as necessary [
28].
1.2. Major Phyla/Class Characteristics of Commercial Microalgal Genera
1.2.1. Chlorophyta
It is a systematic group consisting of green algae that survive in marine contexts, but few are also present in freshwater and on land [
29]. Some microbes can still live in harsh atmospheres such as deserts, saline water, and arctic regions [
30]. These algae appear green due to the huge availability of
chlorophyll, which consists of a
paraphyletic group. Few might appear in colonies consisting of
chlorophyte cells and
apical flagella that engaged during locomotion.
These algae might be asexual or sexual. Asexuality could occur by fission or fragmentation. In comparison, sexuality occurs by exchanging nuclei via conjugation tubes of two identical gametes referred to as
isogamy and
oogamy [
31]. The gametophyte phase is the haploid phase, and the sporophyte phase is the diploid phase. When both the gametophyte and sporophyte phases involve multicellular forms of the species, then it is described as diplobiontic. When only the gametophyte generation is multicellular, it is described as haplobiontic [
32].
1.2.2. Haptophyta
The
Haptophyta or
Prymnesiophyta have 50 genera with more than 500 identified living species [
33]. They are unicellular, live via photosynthesis [
34], and are primarily found in marine and tropical regions, but some are found in freshwater. These algae generally appear to have a golden-brown color due to the presence of
diadinoxanthin and
fucoxanthin, which are yellowish-brown pigments [
35].
1.2.3. Stramenopiles
It is a group of
eukaryotic creatures, occasionally considered as signifying the
phylum (division) of
heterokontophyte, which is proposed based on the latest indication from molecular systematics [
36]. The
stramenopiles include diverse forms, ranging from unicellular (e.g., diatoms) or colonial forms to large multicellular forms, such as brown algae [
37]. Many have been classified into separate
phyla, including the diatoms, brown algae (
Phaeophyta), oomycetes (
Oomycota), golden-brown algae (
Chrysophyta), and yellow-green algae (
Xanthophyta) [
38].
1.3. Microalgae and Their Organization
Algae can survive in diversified regions, from harsh deserts to favorable freshwater lakes, but they need some optimal conditions to grow [
39,
40]. Microalgae have been a source of multidisciplinary products, from pharmaceuticals to daily eating products [
41]. The most impactful utilization of microalgae is effluent treatment and CO
2 eradication [
42]. Its complex structure and cell organization consist of polysaccharides, lipids, pigments, proteins, vitamins, bioactive compounds, and antioxidants [
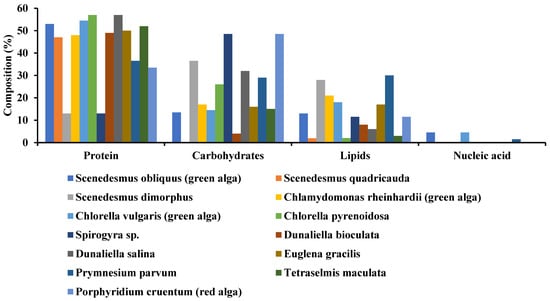
43]. Algae are composed of organic contents such as protein, lipids, nucleic acid, and carbohydrates, but their proportion may vary with the variation in species (See
Figure 1).
Figure 1. Organic composition of some selected microalgae.
Difference between Micro- and Macro Algae
Microalgae and macroalgae are the two main kinds of algae based on cellularity. Microalgae are a unicellular algal genus that may be solitary or live in colonies [
44]. Macroalgae are multicellular algal species [
45]. They are commonly called “
seaweeds” because they can grow profusely anywhere. Microalgae include the
dinoflagellates, the diatoms, and other single-celled algal species [
46].
Both microalgae and macroalgae are essential contributors to atmospheric oxygen through photosynthesis [
47]. They do not have true stems, leaves, and roots. Macroalgae, though, are similarly multicellular, and the cells may function together, forming an organ. The
macroalgal thallus is comprised of the following major parts: (1) the
lamina, (2)
stipe, and (3)
holdfast and
haptera. The
lamina (also called the
blade) is the leaf-like structure, the
stipe is the stem-like structure, and the
holdfast is the root-like structure [
48]. It helps macroalgae to stay afloat. Another floatation-assisting organ is
float. It is located between the
lamina and the
stipe.Microalgae are microscopic, single-celled, and mostly photosynthetic. While uni-celled, a few can create colonies, such as strands or spheres with a similar genus. Their capability to photosynthesize is due to the existence of photosynthetic colorants [
49]. The prevalent colorants inspire the shade of the algae in an algal cell. Thus, they are categorized based on green, red, or brown shades [
50].
Algae are morphologically simple, chlorophyll-comprising, non-flowering, and typically aquatic plants of a large family with members including seaweeds and a range of uni- to multicellular organisms [
51]. They are either
prokaryotes algae with single standard deoxyribonucleic acid DNA in their formation or
eukaryotes with double standard DNA in their makeup and are equipped with a nucleus and chloroplast. Microalgae exist in solitary or chain/group/colony contexts, depending on the species. Their sizes are 3–30 μm, whereas those of the cyanobacteria are 0.2–2 µm [
52].
1.4. Microalgae Cultivation
Microalgae cultivation can be accomplished in open ponds, tanks, raceway ponds, and controlled closed systems [
53]. Extensive research has been conducted on microalgae cultivation [
54,
55,
56]. From the literature, it has been explored that contamination with unwanted microalgae, high bacterial loads, and grazers is common in commercial-scale open-pond systems [
57]. It has also been reported that the damaging impacts of rotifers on the culture
Tetraselmis,
Chlorella,
Nannochloropsis and
Scenedesmus, and
Amoeba also damage diatoms. It is challenging to control the propagation parameters such as evaporation, culture temperature, etc. [
58]. Those are the inherent challenges in implementing large-scale microalgae cultivation in an open-pond system.
Closed cultivation techniques, also called photobioreactors (PBRs) are the most promising approach for achieving quality cultivation because of the highly controlled parameters. This process can be optimized for genes. The extensive light availability decreases the contaminants concerns [
54].
The needs and demands can alter the basic principle of cultivation system design. An open pond is usually built with a circular shape or a gravity-driven flow, whereas the PBRs design has been improvised to accumulate the maximum light. There is limited literature available regarding the two-stage hybrid cultivation system. This cultivation system has been proposed to separate biomass growth from the lipid accumulation phase [
56].
2. Utilization of Microalgae for Wastewater Treatment
2.1. Distillery Waste
Distilleries are among the top industries based on the volume of discharged wastewater. It has been reported that manufacturing a unit liter of ethanol through a distillery process produces more than 10 L of effluent [
64]. The ethanol produced from the distillation step is 8–12% pure, and the residue of this step is called vinasse/spend wash, which is organic. The cleaning and cooling water of the fermenter mixed with the spent wash produces the effluents. The distillery process has been improvised recently, but the economic aspects of effluent treatment are still not appropriately considered.
The effluent consists of rich organic constituents, including polyphenols, organic acids, and recalcitrant compounds such as melanoidins. The Environmental Protection Agency suggests limits for COD and BOD. There is a need for an effective effluent treatment design that is cost-effective to attain the recommended value.
Distillery wastewater treatment is challenging due to its very high organic content and recalcitrant compounds. Due to its recalcitrant and toxic nature, physicochemical processes were initially preferred for the treatment. However, the sludge generation and the cost were found to be the setbacks.
On the other hand, anaerobic, fungal, and thermophilic bacterial treatments were widely preferred. The raw distillery effluent treatment by biological processes was limited due to temporal variations in the loading rate and the inhibitive nature of phenolics and melanoidins constituents. Algal treatment has been emerging as an alternative to conventional treatment. The mixotrophic algal treatment requires less oxygen than other aerobic treatment technologies due to photosynthetic oxygenation.
Distillery waste contains beneficial constituents such as carbon, nitrogen, micro, macronutrients, and vitamins to aid microalgae growth [
65]. Some scientists isolated more than 25 algal strains from the distillery effluents. The algal strains included
Pediastrum sp.,
Scenedesmes sp.,
Perinidinium sp.,
Navicula sp.,
Chroococcus sp.,
Gloeocapsa sp.,
Merismopedia sp.,
Oscillatoria sp.,
Phormidium sp.,
Calothrix sp.,
Syctonema sp.,
Westiellopsis sp.,
Nitzschia sp.,
Spirulina sp.,
Anabaena sp., and
Cylindrospermum sp. [
66].
In a diluted distillery effluent, the
cyanobacterium growth was enhanced compared to a raw distillery effluent. It has been reported that when the effluent is diluted with inorganic media, the maximum biomass of 1.4 g/L was obtained during an algal growth [
67]. This result was also authenticated during the growth of
Chlamydomonas reinhardii in the appearance of vinasse [
68]. The triacetylphosphate media with 1% of vinasse were observed to reach a biomass concentration of 0.543 g/L, whereas without vinasse, it was 0.093 g/L.
Micractinium sp. and
Chlamydomonas biconvexa growths were examined in raw, diluted, and purified vinasse in an Airlift Flat Plate Photobioreactor. It was noticed that the crude vinasse was not helpful for non-axenic algal growth due to fungal contamination and lesser light penetration [
55]. The hydraulic retention time was 74 h with pH 8 to overcome the fungal contamination. The light penetration was increased via dilution or clarification using coagulation [
69].
During the growth, biomass generation was reported at around 0.1 g/L/d in raw vinasse for an axenic culture. In a 50% dilution, the biomass generation in both genes increases to 0.177 g/L and 0.182 g/L, respectively, whereas in a clarified vinasse, it becomes 0.164 g/L and 0.222 g/L [
55].
Chlorella vulgaris was employed in an Anaerobic Fluidized Bed Reactor (AFBR), in which the effluent consisted of 17 g/L of COD and a BOD/COD value of 0.22. OLR was set to 0.14 kg/m
3, with a hydraulic retention time of 10 d [
70]. The removal of COD, BOD, and Phosphorous was 98%, 98%, and 90%, respectively. The recommended source of N for anaerobic digestion was emitted NH
3 during digestion [
71].
Krishnamoorthy et al. attempted to combine membrane technology with a biological approach. They located the microalgae unit of
Oscillatoria sp. among the anaerobic digestor and reverse osmosis (RO) and found that the COD declined up to 55%, reducing the load on RO [
70].
2.2. Heavy Metals
Toxins biosorption and bioremediation, which engages a range of microbes involving yeasts, fungi, microalgae, and bacteria, has developed progressively as a replacement for conventional remedies owing to its ecological and economic advantages [
77]. Microalgae-based bioremediation is an alternative to traditional treatment techniques for cleaning up contamination. The produced biomass has a varied assortment of microalgal biomass utilizations. The production of microalgae has long been utilized to remediate urban effluent [
78].
Some microalgae species, which have extraordinary biologic traits involving high photosynthetic effectiveness and clean composition, can grow well in severe ecological conditions involving intense temperature, nutritional stress, the existence of metallic toxins, and high salinity. Among all microbes, owing to their high binding affinity, the accessibility of a vast number of binding sites, and their enormous surface area, microalgae are rapidly being utilized in the physio-redress of risky toxins [
78,
79].
The most straightforward approach to eradicating metallic toxins is biosorption. Biosorbents can be created from both living and non-living microalgae biomass [
80]. Toxic metals can be carefully eliminated from the ecosystem by using microalgae for bioremediation. Microalgae have an additional benefit over higher plants because they grow rapidly and can be utilized to make biofuels and fertilizers [
81]. Microalgae can also retrieve precious metal ions containing thallium, silver, and gold.
There are only a few reports on the use of microalgae for the biosorption of metals from actual industrial discharges. Freely suspended and restrained
Chlorella vulgaris was shown to be effective in eliminating Fe
+2, Mn
+2, and Zn
+2 from palm oil mill effluent by biosorption [
84]. It was stated that
Spirulina sp. could eliminate residue components, specifically Hg
+2 and Cd
+2, from industrial waste (copper smelter and refinery) by biosorption and bioaccumulation. El-Sheekh et al. [
95] indicated that
Nostoc muscorum and
Anabaena subcylindrical could grow in discharge from salt and soda factories and sewage effluent, eliminating metals such as Cu
+2, Co
+2, Pb
+2, and Mn
+2.
Insight into the Mechanism
Various heavy metals such as Cu
+2, Zn
+2, Ni
+2, Fe
+2, and many others are effectively utilized as micronutrients for microalgae. This metallic content is vital for microalgae cell metabolic activity. But some other heavy metals such as mercury, titanium, cadmium, silver, and gold are not helpful for microalgae growth and behave as toxins for metabolic activity. Microalgae are promising and effective in bioremediation due to outstanding attributes such as survival in harsh environments, the ease of growth, superb binding affinity, effective area, and ecologically friendliness, and dead microalgae can be used for many other purposes [
96].
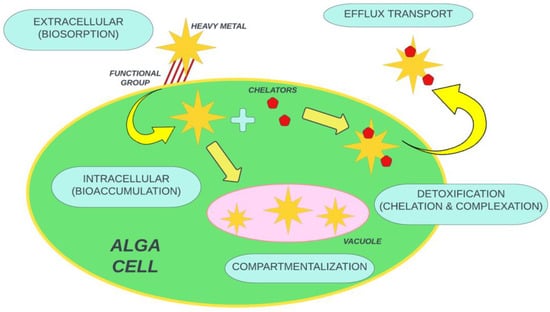
The microalgae mechanism has been elaborated in different dimensions such as gene regulation, chelation, and the desorption of microalgae boost with a decline in pH. The eradication of heavy metal with microalgae is done in a two-step process: (1) rapid, reversible, and passive adsorption onto the cell surface (metallic ions sorb due to electrostatic attraction to the functional group located at the cell wall), followed by (2) a slow, irreversible, active process, involving metallic ion mobility from the cell wall to the cell membrane rather than to
cytoplasm. The initial step occurs in both alive and dead cells, whereas the second step only takes place in living cells [
97]. The eradication mechanism of toxins by the microalgae mechanism is shown in
Figure 2.
Figure 2. General eradication mechanism of toxins by microalgae.
2.3. Textile Waste
Microalgae of unicellular and filamentous genera have been explored for biosorption to remove dyes. Most findings were accomplished utilizing non-viable algal biomass on a dye solution. In one study, the algal biomass of
Microspora sp. after lipid extraction was noticed to be an effective biosorbent for methylene blue, eliminating up to 100% of the dye in 24 h under stirring of 150 rpm [
98].
Defatted
Scenedesmus dimorphus was also assessed for its effectiveness in eliminating methylene blue by biosorption [
99]. The maximum adsorption capacity was analogous to raw and acid-pretreated biomass. Waste residue from the algal biodiesel industry was effective as a biosorbent for dye removal. For instance, it has been testified that biochar derived from
Spirulina platensis after oil extraction for biodiesel was noticed to be an inexpensive biosorbent for methylene blue [
100]. In another analysis, Jing et al. [
101] revealed that biochar derived from the residual biomass of
Ulothrix zonata after pigment extraction might be treated as an affordable biosorbent for malachite green, quartz violet, and Congo red.
Different studies highlighted the potential use of microalgae for the biosorption and bioremediation of textile wastewater and the removal of dyes [
111]. Further, it can be added that marine microalgae are promising candidates for remediating inorganic and organic toxins due to their versatile metabolic activities and microalgae organization.
It has been proven that microalgae are impactful biosorbents for eradicating dyes and other contaminants present in textile waste such as COD, colors, and organic and inorganic toxins. This process has advantages such as economics, a green process, huge availability, and high removal rates with viable process parameters. The optimal result can be achievable by manipulating the parameters such as the microalgae dosing amount, the pH, the temperature, the pretreatment of microalgae, the residence time, and the pollutant concentration in the effluent.
2.4. Emerging Pollutant
EPs are identified as threats to the environment, health, and living beings. Many approaches have been utilized recently to eradicate EPs from the effluent, but adsorption is the most economical and easiest way to deal with such toxins. Recently, microalgae have received much attention due to their capability to eradicate many toxins, including EPs such as pharmaceutical contents, personal care products, and non-pharmaceutical contents present in effluent, due to the economic, easy, and innovative solution in comparison with traditional approaches [
114]. Microalgal materials have been used to eradicate EPs. It was found that they were much better than other conventional processes.
2.4.1. Pharmaceuticals
Pharmaceutical-based EPs eradication has become a significant concern, and researchers have focused on its remediation approach. Microalgae have been considered as an adsorption agent for removing these toxins. However, the research was mostly conducted on a lab scale with optimum conditions, but it is the demand of the current era to scale up this approach for the eradication of toxins commercially. However, the industrial application of microalgae for the remediation of EPs is still unclear because of a significant gap between lab outcomes and commercial applications.
2.4.2. Non-Pharmaceuticals
Industrial effluent can contain substances that limit microalgae growth. When this issue persists, it becomes a challenge because the kind of effluent and its constituent can inhibit the eradication process; for example, olive mill effluent has antibacterial properties with phytotoxicity due to the high amount of phenolic content [
122].
Researchers have attempted to deal with such challenges, and it was noticed that genetically adaptive microalgae could survive in harsh effluents such as herbicides, mining effluent, antibiotics, and many other toxins [
20]. Studies reveal that preliminary microalgae exposure could eradicate cefradine better than wild genes [
123]. In other findings, it has been reported that acclimated microalgae strains could be favorable to raw effluent.
Chlorella luteoviridis and
Prachorella kessleri were acclimated to municipal effluent within an acclimation duration of 2 months [
124]. The acclimation to effluent tolerance was interlinked with an accumulation of carotenoid pigments. It was enhanced with ascorbate peroxidase activity. Isolated
P. kessleri from effluent revealed good capabilities to survive in a diversified atmosphere [
125].
The major problem faced during EPs eradication was the appearance of EPs in tiny concentrations. It was observed that the EC
50 (the concentration of ECs at which 50% of microalgae growth is inhibited) was very high and had a magnitude greater than the ECs concentration in the effluent [
126]. It was authenticated previously in 2016 by a group of researchers when they employed
Chlorella vulgaris inhibited by diazinon with a maximum eradication of 94% at 20 mg/L [
127].
Biperiden and Trihexyphenidyl were eradicated by
Coelastrella sp. with 92% and 94% effectiveness, respectively. Bioremediation/biosorption took place due to the biotransformation of complexes into simple units. Compared with biosorption or bioaccumulation, the biodegradation process can deal with such complex EPs by facilitating the complexes inside the algal cells [
137]. Hence, it can be assumed that the EPs eradication using microalgae species is quite time-consuming, with huge algal sludge formation and sensitive parameters control. Besides all these concerns, there is a chance to compete with the conventional approaches of eradicating EPs due to the cheaper, economical, viable, and limited utilization of hazardous chemicals for treatment.
This entry is adapted from the peer-reviewed paper 10.3390/fermentation9030311