Many causes and risk factors contribute to the overarching incidence of male infertility, graded as idiopathic, acquired and congenital [
3]. Previous reports estimate that about 30–50% of male infertility patients are idiopathic [
2,
4]. In addition, infertile couples experience many emotions, including depression, anger and shame. Furthermore, they are sometimes mocked, embarrassed and even pressured by peers, friends and parents, particularly in societies with high expectations for bearing children after marriage [
4]. Acquired factors of male infertility include varicocele, testicular trauma, testicular torsion, recurrent urogenital infections and acquired secondary hypogonadism [
5]. Among these factors, varicocele is the most common cause of infertility in men, with a prevalence of 40% [
5]. In addition, congenital causes of male infertility arise from the congenital bilateral absence of the vas deferens associated with cystic fibrosis, gene mutations and chromosomal abnormalities leading to the deterioration of testicular function and Y chromosome microdeletions resulting in isolated spermatogenic defects [
6]. These risk factors may also cause excessive accumulation of reactive oxygen species (ROS) in cells and induce damage to the reproductive function by releasing inflammatory cytokines and, eventually, oxidative stress [
7]. ROS affect sperm parameters directly or indirectly by impairing male reproductive hormones, cells and organs [
7].
Some factors leading to male infertility can be surgically reversed or therapeutically ameliorated with drugs [
8]. However, treatment options depend on the cause of male infertility, the patient’s age, financial status, facilities available in a designated hospital and expertise [
9]. The primary treatment for male factor infertility is intrauterine insemination (IUI), tubal and male ejaculatory duct cryosurgery, in-vitro fertilisation (IVF) and embryo transfer with or without intracytoplasmic sperm injection (ICSI) [
9]. In addition, psychosexual counselling, vacuum constriction device (penis pump), diet, exercise, weight loss, approved phosphodiesterase type 5 (PDE5) inhibitors drugs, including Viagra (Sildenafil), Cialis (Tadalafil) and Levitra (Vardenafil) and Stendra (Avanafil), apomorphine and intracavernosal injection therapies as well as medicinal plants, such as Pausinystalia yohimbe and Tribulus terrestris, are used for the treatment of erectile dysfunction [
8,
10]. Furthermore, testosterone replacement therapy (TRT), aromatase inhibitors and human chorionic gonadotrophin (hCG) therapy [
11,
12] are used for the treatment of primary hypogonadism. Most of these male infertility treatment options have been deemed adequate. However, they also have their downsides regarding side effects, costs, invasiveness of the administration method and availability, particularly in African countries [
8,
9,
11,
12]. Therefore, medicinal herbs may be recommended to treat male reproductive impairment, as these herbs enhance reproductive functions [
13,
14].
2. Male Infertility
There is increasing evidence of the progressive decline in human fertility in both developed and developing countries, with a variable prevalence [
2]. For instance, the prevalence estimate of infertility is 6% in the United States of America (USA), 10–15% in the United Kingdom (UK) and 20–46% in sub-Saharan Africa, with the African continent having an overall infertility rate of 41.91% among males and females, in which male infertility contributes to 22.26% [
3,
6,
24].
Male infertility is commonly caused by reduced semen quality characterised by low sperm count (oligozoospermia), impaired motility of the sperm (asthenozoospermia), decreased vitality of the sperm (necrozoospermia), impaired morphology of the sperm (teratozoospermia) or a combination of these parameters termed oligoasthenoteratozoospermia and azoospermia [
2]. In total, 90% of male infertility problems are linked to sperm count, and there is a link between reduced sperm count and reduced semen quality [
6]. In addition, a cohort study of South African men of reproductive age indicated in 34.2% of the sub-fertile men, 11.9% had severe azoospermia [
25].
Several risk factors can disrupt semen quality leading to semen-related abnormalities [
6]. Oxidative stress has been linked to male infertility due to enhanced reactive oxygen species (ROS) production or decreased antioxidants. ROS leads to infertility through two fundamental mechanisms. Firstly, by inducing sperm membrane damage, which decreases sperm motility and its fertilisation capacity, as well as by altering the DNA molecule of sperm, resulting in the passage of defective paternal DNA on to the conceptus [
7].
3. Oxidative Stress
Oxidative stress (OS) refers to an imbalance between the production and accumulation of ROS and the biological system’s ability to detoxify these ROS in cells and tissues [
26]. These ROS are radical and nonradical oxygen species that form through partial oxygen reduction within the mitochondria [
27]. Oxidative stress contributes to multiple pathological conditions and diseases [
28]. For example, moderate oxidative stress may cause cell dysfunction and altered behaviour, such as accelerated senescence, abnormal proliferation, dysregulated inflammatory responses and cell tumorigenesis. In contrast, high OS usually causes cell death (e.g., oncosis, apoptosis and autophagy) [
29].
Cells have an antioxidant defence system that is made up of enzymes (e.g., superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione reductase (GR) and non-enzymatic molecules (e.g., ascorbic acid, tocopherol, carotene) that can neutralise or scavenge ROS [
30]. However, with the abundance of exogenous and endogenous sources of ROS, reproductive cells, in particular, lose the ability to balance the levels of oxidants and antioxidants over time, leading to damage to cellular constituents, such as lipids, DNA and proteins [
31,
32], which further complicates the functions of the reproductive cells and lead to male infertility [
33].
For instance, OS is a significant cause of sperm cell dysfunction and contributes to the aetiology of male infertility due to the impairment of both spermatozoa’s structural and functional integrity [
34,
35]. It disrupts the integrity of the DNA because of concurrent damage to proteins and lipids present in the sperm cell plasma membrane, affecting cell membrane fluidity and permeability [
36]. The presence of high levels of DNA damage in human spermatozoa has been correlated with adverse clinical outcomes, including recurrent pregnancy loss, dominant genetic disorders and infertility.
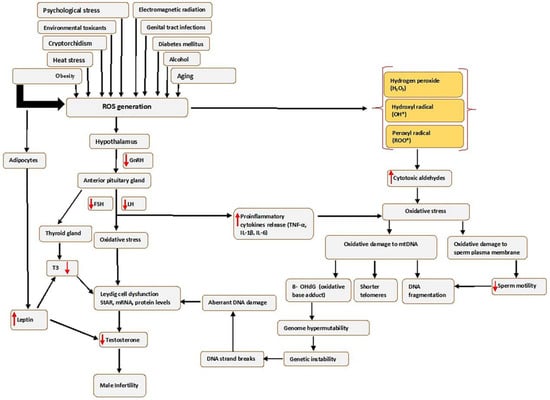
Oxidative stress affects sperm function in two ways: by damaging the sperm nuclear and mitochondrial DNA (mtDNA), which is associated with shorter telomere length, formation of the oxidative base adduct 8-hydroxy-deoxyguanine (8-OHdG) and fragmentation of mitochondrial DNA; or by damaging the sperm plasma membrane and thus affecting sperm motility and its ability to fuse with the oocyte often leading to genome hypermutability, genetic instability, single-strand and double-strand breaks, aberrant DNA damage, Leydig cell dysfunction and finally leading to infertility (see Figure 1).
Figure 1. Causes of seminal oxidative stress and oxidative DNA damage. Various factors can lead to or affect the generation of reactive oxygen species (ROS) in the male germ line, which creates oxidative stress. Abbreviations: FSH-Follicle stimulating hormone; IL-6-interleukin 6; LH-luteinizing hormone; mRNA-messenger RNA; ROS, Reactive oxygen species; StAR-steroidogenic acute regulatory protein; TNF-α-Tumour necrosis factor alpha; T3, Triiodothyronine; 8 OHdG-8-hydroxy-2′-deoxyguanosine. Red arrows pointing up indicates increase; red arrows pointing downwards indicates a decrease.
Furthermore, the risk factors that increase ROS in the male genital tract can create an imbalance in the production of oxidants and the scavenging capacity of antioxidant enzymes, consequently leading to OS, as illustrated in
Figure 1. In addition, high levels of ROS may disrupt the hormonal balance that regulates male reproductive functions by acting on the hypothalamic–pituitary–gonadotropic (HPG) axis, thus reducing the secretion of LH and FSH from the anterior pituitary gland [
37]. The reduced LH secretion results in failure to stimulate Leydig cells to produce sufficient testosterone [
38], while reduced FSH negatively affects the release of androgen-binding protein (ABP), which helps in concentrating testosterone. This causes an overall decrease in Leydig cells’ function and, in turn, affects the proteins mediating cholesterol uptake into the mitochondria, such as the steroidogenic acute regulatory (StAR) protein, or by increasing concentrations of inflammatory cytokines [
39], and consequently decreasing circulating testosterone [
40], which results in unregulated spermatogenesis and suppression of sexual behaviour [
7].
In addition, obesity results in the excessive production of ROS by stimulating the adipocyte cells to produce leptin, the critical regulatory adipokine [
41]. The increased leptin secretion may also decrease testosterone production by the Leydig cells through altering the endocrine regulation [
42], which is mediated primarily through the hypothalamic–pituitary–gonadal (HPG) axis [
43], which affects the release of hypothalamic GnRH, FSH and LH. This will activate OS through cellular metabolisms, negatively impacting the differentiation processes of germ cells. The obesity-induced testicular OS explains this scenario.
Moreover, obesity may induce testicular OS via other potential mechanisms, such as increased fatty acid oxidation in mitochondria and peroxisomes by adipose tissue, which may lead to a higher generation of ROS. Thus, more elevated ROS mediates oxidative damage to biomolecules that include lipids, proteins and DNA. These cause oxidation of polyunsaturated fatty acids in the sperm membrane, loss of the mitochondrial membrane potential and single- and double-strand sperm DNA fragmentation (SDF) [
44]. OS may also impact the hypothalamic–pituitary–thyroid (HPT) axis by decreasing the secretion of triiodothyronine (T3) and triiodothyronine (T4). Reduction in T3 lowers the levels of StAR, mRNA and protein in Leydig cells and reduces the generation of testosterone [
45].
Spermatozoa are susceptible to OS because their plasma membrane contains an abundance of polyunsaturated fatty acids (PUFAs). The PUFAs in sperm are required to create fluidity, which is crucial for sperm motility, acrosome reaction and egg fertilisation [
46]. An increase in unsaturated fatty acid content is associated with ROS generation that results in a decline in sperm motility [
47] either through the ability of H
2O
2 to diffuse across the membranes and inhibit the activity of several enzymes crucial for the sperm movement [
48], or through inhibition of phosphorylation of axonemal proteins and subsequent sperm immobilisation [
49]. High levels of ROS in the spermatozoa perpetuate a lipid peroxidation (LPO) cascade and ultimately drive these cells into a state of oxidative disintegration of DNA and proteins [
50].
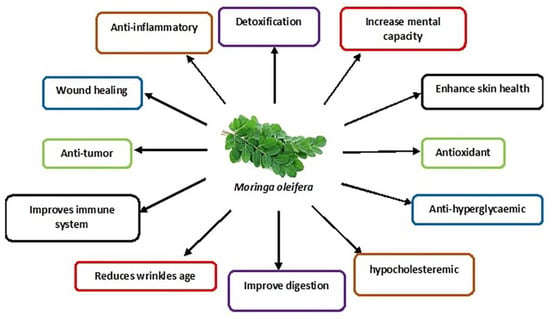
4. Moringa oleifera
The
M. oleifera tree is mainly cultivated for its various uses as an essential herb due to its nutraceutical and medicinal properties [
62]. The different parts of the tree, such as the roots, flowers, fruits, seeds and leaves, are traditionally used to treat abdominal tumours, hysteria, scurvy, paralysis, helminthic bladder, prostate problems, sores and skin infections [
19]. In addition, its leaves are commonly used as they contain many bioactive compounds such as nutrients and phytonutrients [
63]. The properties of
M. oleifera leaves are summarised in
Figure 2.
Figure 2. Schematic diagram showing various medicinal uses of M. oleifera leaves.
These nutrients include proteins, vitamins (E, C, beta-carotene, B-6), minerals (calcium, phosphorus, magnesium etc.) and fatty acids [
64]. The intrinsic bioactive phytonutrients include flavonoids, phenolic acids and glycosides. Other groups include saponins, alkaloids, tannins, isothiocyanate and glucosinolate [
17]. The flavonoid group mainly include quercetin and kaempferol, with a concentration of up to 137.81 and 106.75 mg/g, respectively, found in the
M. oleifera leaves. The phenolic acid in the
M. oleifera leaves includes ferulic acid, gallic acid, vanillic and ellagic acid, with chlorogenic acid being the most abundant [
14,
65].
M. oleifera leaves show significant protective effects against many diseases [
16] and the widely persistent environmental toxins that disrupt cellular metabolic function [
66].
M. oleifera is even used to treat neuro-dysfunctional diseases such as Alzheimer’s, ischaemic stroke and epilepsy [
14]. Studies have also confirmed its potential treatment in chronic diseases such as diabetes mellitus (insulin resistance and hyperglycaemia) [
67] and high blood pressure [
64]. In addition,
M. oleifera is a preventive strategy for various conditions and diseases, e.g., preventing testosterone-induced benign prostate hyperplasia [
67]. Furthermore,
M. oleifera has been used to enhance male sexual functions, including libido, erectile dysfunction and testicular injury [
14,
68], the symptoms of primary hypogonadism that mostly leads to male infertility.
5. Studies on the Effects of M. oleifera Leaf Extracts on Male Reproductive Function
5.1. Sperm Parameters
M. oleifera significantly affects the improvement of sperm characteristics; this is demonstrated by the Egyptian buffalo bulls fed with 4% and 8% concentrations of
M. oleifera leaves in their diet [
69]. Furthermore,
M. oleifera has been shown to reduce sperm abnormality in male Swiss mice fed with 4% and 8%
M. oleifera in their diet [
70]. In addition, rabbit bucks administered with 200 and 400 mg/kg B.W. showed an increase in sperm viability, sperm membrane integrity and sperm motility [
20]. Finally, Bali bulls fed with a diet containing 15%
M. oleifera demonstrated increased sperm motility [
14].
5.2. Hormonal Levels
M. oleifera significantly increased serum testosterone levels and gene expressions for luteinizing hormone and follicle-stimulating hormone in Rabbit bucks [
20]. However, in buffalo bulls [
69] and Bali bulls [
14], there is only an increase in testosterone with no effect on LH and FSH. Contrastingly,
M. oleifera leaf extract increased the concentrations of FSH and LH in New Zealand white (NZW) rabbit bucks. The extracts also increased semen volume, sperm count and motility [
71].
5.3. Testis
Administration of
M. oleifera improved the testicular structure of rabbit bucks. High doses of moringa leaves led to germinal hyperactivity of cells, such as the increase in the number of cells at all stages of spermatogenesis, increased density of spermatids, prominence of spermatozoa in the lumen of seminiferous tubules and wider interstitial areas with normal Leydig cells compared to non-supplemented rabbit bucks [
20]. Additionally, a significant increase in seminiferous tubule diameter, height and epithelium area, type A spermatogonia and spermatogenic efficiency, as well as the increased number of Sertoli cells and total spermatogenic cells was demonstrated following treatment in male rats [
72]. In addition, a significant increase in relative testis, epididymis and seminal vesicle weight, and diameter of the seminiferous tubules was observed in male-treated mice [
21].
5.4. Male Reproductive Cells: Leydig Cells and Sperm Cells
M. oleifera leaf extracts (10, 50, 100, 250, 500 and 1000 ug/mL) on TM3 Leydig cells increased the levels of testosterone under stimulatory conditions of hCG. However, the testosterone increase was seen only in 500 and 1000 ug/mL concentrations under basal conditions [
22]. In addition,
M. oleifera also demonstrated its antioxidative effects by increasing the glutathione concentration in the cells exposed to 250 ug/mL of the extract [
22].
M. oleifera leaf extracts were also observed on sperm cells with varying concentrations (0.625; 6.25; 62.5; 625 ug/mL). The findings indicated that
M. oleifera inhibited the formation of sperm intracellular ROS at 62.5 and 625 ug/mL, reduced the percentage of sperm with DNA fragmentation and increased the percentage of incapacitated and intact acrosome spermatozoa at 625 ug/mL [
73].
6. Effects of M. oleifera Leaf Extracts on Male Reproductive System Constituents following Exposure to Male Infertility Risk Factors
Table 1 summarises the effect of the M. oleifera on the male reproductive system following exposure to various infertility risk factors.
Table 1. Effects of M. oleifera leaves on damage induced by male infertility risk factors in the male reproductive system.
|
Male Infertility Risk Factors
|
Oxidative Stress Parameters
|
Hormonal Levels
|
Sperm Parameters
|
Gene Expression
|
Testicular Histology Examination
|
Authors
|
|
Heat stress
|
↑ TAC,
↓ GST
|
↑ Testosterone levels
|
↑ sperm quality (sperm concentration with intact acrosome, total sperm output, motility and viability).
|
|
↑ the normal morphology and number of the tubular epithelial cells, germinal Sertoli cells, spermatogonia, spermatocytes, early spermatids, late spermatids and spermatozoa.
↓ Leydig cells and Sertoli cells pyknosis.
|
[74,75,76,77]
|
|
Electromagnetic radiations
|
↓ MDA, ↑ SOD and
↑ CAT
|
↑ Serum testosterone levels
|
↑ Epididymal sperm count and motility.
↓ sperm defects (pyriform head, detached head, coiled tails and multiple abnormalities).
|
|
↓ degeneration in some parts of the seminiferous tubules and
↑ the number of Leydig cells.
|
[78,79,80]
|
|
Environmental toxicants
|
↓ testicular tissue GST activity and MDA.
↑ GPx.
↑ SOD,
↑ CAT
|
↑ Testosterone. ↑ serum FSH and LH
|
↑ sperm motility, ↑ sperm viability, ↑ sperm count and ↓ sperm abnormalities.
|
↑ StAR gene,
↑ cytochrome p450o17 subfamily A (CYP17A),
↑ CYP11A1 and
↑ HSD17B3 genes of the steroidogenic hormones.
↓ expression of CYP19A1 aromatase gene.
|
↓ weight of the reproductive organ.
↑ elongated spermatids and spermatozoa,
↑ the epididymal histological integrity,
↑ sperm density and
↓ congestion and interstitial oedema in the seminal vesicle and prostate gland.
|
[81,82,83,84,85]
|
|
Obesity
|
↓ MDA,
↑ SOD,
↑ CAT and ↑ GSH
|
↑ Testosterone, ↑ FSH and
↑ LH
|
↑ sperm count, ↑ sperm motility, ↓ immotile spermatozoa,
↓ primary and secondary sperm abnormalities.
|
|
|
[86,87]
|
|
Diabetes
|
↓ TBARS,
↑ SOD,
↑ CAT,
↑ GSH and
↑ Ascorbic acid
|
↑ LH,
↑ FSH and
↑ testosterone
|
↑ sperm count and
↑ sperm mobility.
|
|
↑ mean number of spermatogonia in the seminiferous tubules, ↑ population of the round (normal) spermatids.
↑ diameter of the seminiferous tubules,
↑ nuclear diameter of the Leydig cells and
↑ weight of the epididymis.
|
[88,89]
|
|
Therapy and medications (HAART)
|
|
↑ FSH,
↑ LH and
↑ testosterone
|
↓ the sperms with abnormal morphology,
↑ semen quality (sperm progressivity, sperm volume, sperm motility, sperm count and viability).
|
|
↑ testicular weight.
↑ normal testicular morphology.
|
[90]
|
|
Alcohol
|
|
|
|
|
↑ myoid living cells, spermatogenic living cells, spermatogonia, spermatocytes, spermatids and spermatozoa and lumen filled with semen.
↓ Reduced Leydig cells disruption
|
[91]
|
|
Psychological stress
|
|
↓ PDE-5 activity,
↑ testosterone and
↓ corticosterone
|
|
|
↑ interstitial Leydig cells and
↑ spermatozoa in the seminiferous tubule lumen.
|
[92]
|
|
Aging
|
|
|
↑ sperm count and
↑ normal sperm morphology.
|
|
|
[93]
|
|
Cryptorchidism
|
↓ GGT activity,
↑ SOD activity and
↓ MDA
|
↑ testicular testosterone
|
↑ sperm count,
↑ germ cell count.
|
|
↑ testicular weight,
↓ the abnormal appearance of the testes.
↓ abnormal appearance of the seminiferous epithelium.
|
[94,95]
|
|
Food
|
|
|
↑ sperm motility.
|
|
|
[96]
|
↑ = Increase; ↓ = Decrease. Abbreviations: CAT, catalase; GST, glutathione -S-transferase; GGT, gamma-glutamyl transferase; HAART, highly active antiretroviral therapy; FSH, follicle stimulating hormone; GSH, glutathione; GPx, glutathione peroxidase; LH, luteinizing hormone; MDA, malondialdehyde; PDE-5, Phosphodiesterase-5; SOD, superoxide dismutase; StAR, steroidogenic acute regulatory protein; TAC, total antioxidant capacity; TBARS, thiobarbituric acid reactive substances.
7. Mechanism of Action of M. oleifera Extract on Oxidative Stress and Male Fertility
Moringa oleifera extracts possess anti-oxidative, anti-inflammatory, anti-diabetic, anti-obesity and anti-apoptotic properties [
86,
126], which have been attributed to its polyphenols, flavonoids (particularly quercetin and kaempferol), phenolic acids, caffeoylquinic acid and isothiocyanates [
127,
128]. The alkaloids, flavonoids, saponins, triterpenoids/steroids and tannins in
M. oleifera extract are powerful anti-oxidants that acts to prevent new free radicals and chain reactions and protects the cells from oxidative damage [
129].
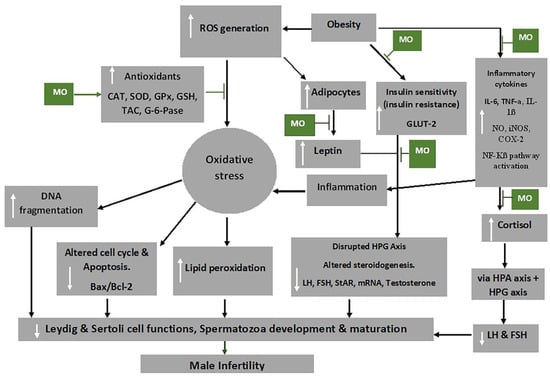
Figure 3 shows some mechanisms through which
M. oleifera extract inhibit the damaging effects of oxidative stress, thereby preventing male infertility.
Figure 3. Mechanism of action of
Moringa oleifera (MO) extract on oxidative stress and male infertility. Abbreviations: CAT-catalase; FSH-follicle stimulating hormone; COX-2-cyclooxygenase -2; GLUT2-glucose transporter 2; G-6-pase-glucose 6-phosphatase; GPx-glutathione peroxidase; GSH-glutathione; HPA-hypothalamic–pituitary–adrenal axis; HPG-hypothalamic–pituitary–gonadal axis; IL-6-interleukin 6; iNOS-inducible nitric oxide synthase; LH-luteinizing hormone; mRNA-messenger RNA; NF-Kβ-nuclear factor kappa-light-chain-enhancer of activated B cells; NO-nitric oxide; ROS-reactive oxygen species; SOD-superoxide dismutase; StAR-steroidogenic acute regulatory protein, TAC-total anti-oxidant capacity; TNF-α-tumour necrosis factor alpha. White arrows pointing up indicates increase; white arrows pointing downwards indicates a decrease.

Indicated the regulatory/inhibitory role of Moringa oleifera extract.
Obesity activates the adipocytes to further produce leptin and could lead to leptin resistance, which has been shown to inhibit the GnRH neurons due to the suppression of KISS1 neuron activities and increased NPY levels [
130]. This, consequently, affects the HPG axis, by the impairment of the release of GnRH, FSH and LH, and ultimately impairs the functions of the reproductive cells, testosterone release and development and maturation of the spermatozoa [
131]. Besides the reduction of food intake, the anti-obesity and anti-hyperglycaemic activities of
M. oleifera are brought about by the reduction of leptin levels by down regulating mRNA expression of leptin and resistin [
132]. Additionally, hyperglycaemia may occur due to oxidative stress associated with obesity as it impedes the functioning of insulin and glucose utilisation by peripheral tissue [
133]. Flavonoids, particularly quercetin, in the extracts has been shown to act as an apical inhibitor of glucose transporter 2, demonstrating its anti-hyperglycaemic activity [
134]. Furthermore, the anti-hyperglycaemic activity of the plant extract is noted by the inhibition of α-glucosidase, pancreatic α-amylase and intestinal sucrose [
135].
Inflammatory cytokines, including IL-1β and TNF-α, can increase the production of prostaglandin E2 (PGE-2), nitric oxide (NO), inducible NO synthase (iNOS), cyclooxygenase-2 (COX-2) and microsomal PGE synthase-1 (mPGES-1) as well as their expression in target cells [
136].
M. oleifera has also been shown to reduce the production of inflammatory cytokines, such as of TNF-α and IL-6 [
126], as well as inhibit the expression of RelA, a gene involved in NF-kB p65 signalling during inflammation [
137].
Cortisol and leptin, respectively, produce a primary and secondary negative feedback mechanism for the HPA axis [
138], which is critical in maintaining equilibrium under stressful conditions. Chronic psychological stress, for instance, can bring about dysregulation of cortisol [
139]. The high levels of ROS result in the release of cortisol (stress hormone), which is usually activated by the HPA axis. The HPA axis, through its communication with the HPG axis, decreases the release of LH, FSH and, ultimately, testosterone [
38,
140]. In addition, the HPT axis is also affected by oxidative stress and consequently decreases T3 production from the thyroid gland and decreases the circulating testosterone through HPT–HPG axes cross-talk [
7].
High levels of ROS disrupt the inner and outer mitochondrial membrane by the induction of cytochrome c protein and activation of caspases and apoptosis [
141].
Moringa oleifera extract down regulates caspase 3 and the activation of pathways of NF-kB and phosphatidylinositide 3-kinase/protein kinase B (P13K/AKT) and suppress testicular apoptosis by down regulating Bax expression [
70,
142,
143], thereby preventing male infertility. NF-kB promotes the transcription of genes engaged in apoptosis of male germ cells, which may result from stimulation of Bax/Bcl2 and initiation of caspases [
144]. In addition, it has been demonstrated that mechanisms that reduce ROS production ensure that the Bcl-2 inhibitor gene of apoptosis protects the cells [
141].
 Indicated the regulatory/inhibitory role of Moringa oleifera extract.
Indicated the regulatory/inhibitory role of Moringa oleifera extract.