Metal nanoclusters (NCs), comprising only a few to roughly hundreds of metal atoms, have a metal core-protective agent shell structure. Owing to the size of metal NCs approaching the Fermi wavelength of electrons, the spatial confinement of free electrons in metal NCs generates discrete electronic transitions, thereby exhibiting intriguing molecular-like properties. Therefore, metal NCs are deemed to bridge the gap between molecules and nanoparticles.
- nanomaterials
- electrochemiluminescence
- fluorescence
Note:Dear author, the following contents are excerpts from your papers. They are editable. And the entry will be online only after authors edit and submit it.
Definition:
Metal nanoclusters (NCs), including Au, Ag, Cu, Pt, Ni and alloy NCs, have become more and more popular sensor probes with good solubility, biocompatibility, size-dependent luminescence and catalysis. The development of electrochemiluminescent (ECL) and chemiluminescent (CL) analytical methods based on various metal NCs have become research hotspots. To improve ECL and CL performances, many strategies are proposed, from metal core to ligand, from intermolecular electron transfer to intramolecular electron transfer. Combined with a variety of amplification technology, i.e., nanostructure-based enhancement and biological signal amplification, highly sensitive ECL and CL analytical methods are developed.
1. Introduction
Metal nanoclusters (NCs), comprising only a few to roughly hundreds of metal atoms, have a metal core-protective agent shell structure. Owing to the size of metal NCs approaching the Fermi wavelength of electrons, the spatial confinement of free electrons in metal NCs generates discrete electronic transitions, thereby exhibiting intriguing molecular-like properties. Therefore, metal NCs are deemed to bridge the gap between molecules and nanoparticles. In recent years, they have attracted a great deal of research in the applications of cellular imaging and chemical/biological detection owing to small size, excellent biocompatibility, good stability, distinctive catalytical activity, optical and electrochemical properties [1,2,3,4,5,6,7].
ECL phenomenon of fluorescent Ag NCs was studied for the first time in 2009 [8]. In 2011, Chen’s group and Zhu’s group found that Au NCs/triethylamine (TEA) system and Au NCs/potassium persulfate system had ECL phenomenon, respectively [9,10,11]. Based on these, they established new methods for the determination of Pb2+, dopamine and hydrogen peroxide. Subsequently, Yuan et al. realized the highly sensitive detection of phenols, microRNAs and dopamine with Au, Ag, and Cu NCs as ECL probes and potassium persulfate or hydrazine as coreactants [12,13,14]. Up to now, metal NCs, such as Au, Ag, Cu, Pt, Ni and alloy NCs have been widely studied as luminophores, catalysts, or quenchers in ECL system. Among them, there are many studies on luminophores. Unfortunately, the ECL efficiency of metal NCs is usually low, which significantly limits their sensing capability and application. To solve this problem, many strategies, such as valence state regulation [15,16], peroxidation [17], host-guest recognition [18], tuning ligand effects [19], covalent bonding of coreactants [20], have been reported recently. Many reviews have reported the ECL of metal NCs [21,22,23,24,25,26], but there is no systematic review on the methods to improve the luminescent efficiency of metal NCs.
Compared with ECL, metal NCs have been considered in CL applications since 2011 [27]. The CL signal of conventional molecular systems is often weak due to low quantum efficiency. Metal NCs could possess catalytic activity [28,29]. Therefore, the catalytic activity of metal NCs in CL system has been studied widely. In order to gain better analytical performance, some valuable researches have been done to improve catalytic activity of metal NCs, i.e., alloy NCs, synergistic effect of carbon nanomaterials and so on [30,31,32]. However, there are few studies on metal NCs as luminophores or quenchers [33,34,35,36,37].
2. Electrochemiluminescence of Metal NCs
Since the ECL property of fluorescent Ag NCs was reported in 2009 for the first time [8], both anodic and cathodic ECL applications of metal NCs have been widely studied. These researches revealed versatile metal NCs, such as luminophores, catalysts and quenchers, and provided more possibility in the discovery of novel highly efficient ECL systems.
2.1. Metal NCs as ECL Luminophores
Au NCs have become the most investigated metal NCs in ECL applications. Studies of the ECL performance of other single metal NCs and alloy NCs are still in the preliminary stages and success in raising ECL efficiency has been limited. The common synthesis strategies of metal NCs are to choose appropriate templates and reducing reagent or templates with reductive properties, such as lipoic acid (LA), bovine serum albumin (BSA), methionine, glutathione (GSH), N-acetyl-L-cysteine, and DNA, to form metal NCs. However, the relatively low ECL efficiency of metal NCs has restricted their further applications. Another hurdle to the improvement of ECL efficiency is the unclear mechanism of the ECL, which prevents the innovation of new metal NCs.
To date, a large number of efforts have been made to improve the ECL efficiency of metal NCs. Many studies are illustrated from the following perspectives: metal core valence states of metal NCs, bimetallic core, single metal core doped with electron rich rare earth elements, the number and category of template, coreactant, coreaction accelerator, synergistic effect and plasmon resonance enhancement effect of nanomaterials, nanostructure substrates, and bio-signal amplification effect (Figure 1). In addition, the researchers realized the intramolecular electron transfer by covalently linking luminophores and coreactants or luminophores, coreactants and coreaction accelerators, which differed from the original inefficient intermolecular electron transfer. Furthermore, a lot of researches have adopted a combination of two or three methods instead of a single approach to improve ECL efficiency, for example the combination of the intramolecular electron transfer and target-catalyzed hairpin hybridization amplification strategy [46].
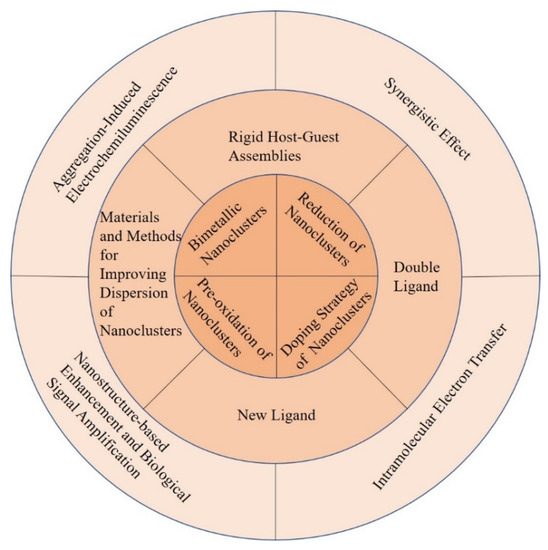
Figure 1. Schematic representation of the strategies for improving the ECL efficiency of metal NCs.
2.2. Metal NCs as ECL Quenchers
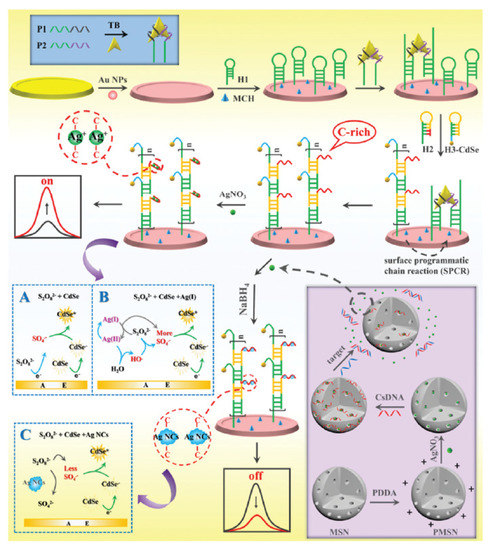
Figure 6. Schematic diagram of the “on–off” ECL biosensing platform for versatile detection of thrombin and miRNA-21 based on Ag(I) ion accelerated and Ag NC-quenched ECL combined with molecular machine-triggered chain reaction and MSN double amplification. (A) The ECL process of CdSe QDs + S2O82− system; (B) The ECL process of CdSe QDs + S2O82− system with Ag(I) ions as coreaction accelerators; (C) The ECL process of CdSe QDs + S2O82− system with Ag NCs as acceptors and CdSe QDs as donors. Reprinted from [76] with permission from RSC.
2.3. Metal NCs as ECL Catalysts
Luo et al. proposed a sensitive ECL biosensor for detection of protein kinase activity (PKA) based on the BSA/Au NCs enhanced cathodic ECL of graphite-like carbon nitride material (g-C3N4)-S2O82− system [78]. After dropping the g-C3N4 onto the surface of a glassy carbon electrode (GCE), peptides were assembled by reaction with chitosan. Using PKA as a model kinase, BSA/Au NCs were immobilized onto the phosphorylated peptides modified g-C3N4/GCE by Au-S bond in the presence of adenosine 5′-[γ-thio] triphosphate (ATP-s) and PKA. The assembled BSA/Au NCs as the catalysts of the cathode ECL reaction of g-C3N4 can significantly enhance the ECL intensity. The resultant ECL signal of g-C3N4 was magnified 4.5 times compared with the absence of BSA/Au NCs. The proposed ECL platform is capable of analysing PKA activity of biological sample quantitatively and screening kinase inhibition qualitatively.
Pt NCs play double roles of the acceptor and the donor in the multiple ECL resonance energy transfer (RET) system [79]. The PL emission spectrum of the Alexafluor (AF) overlapped with both the UV-Vis absorption spectra of polyethyleneimine stabilized Pt NCs and tris(4,4′-dicarboxylic acid-2,2′-bipyridyl) ruthenium(II) dichloride (Ru(dcbpy)32+). It is indicated that resonance energy transfer (RET) can be generated not only between AF and Pt NCs but also between AF and Ru(dcbpy)32+. Then the PL emission spectrum of the Pt NCs overlaps with the UV-vis absorption spectrum of Ru(dcbpy)32+, displaying the presence of RET between Pt NCs and Ru(dcbpy)32+. Therefore Pt NCs can accept energy from alexafluor, transfer it to Ru(dcbpy)32+ and then enhance ECL efficiency. The existence of multiple energy donor/acceptor pairs in the same nanostructure resulted in a shorter electron-transfer path, less energy loss and higher RET efficiency. Combined with target recycling amplification technology, the ECL efficiency is 1.78 times higher than the classic Ru(bpy) 32+.
3. Chemiluminescence of Metal NCs
3.1. Metal NCs as CL Catalysts
The applications of metal NCs in CL mainly focus on the catalysis, and the research on metal NCs as luminophore and quencher is less. Metal NCs are served as catalysts based on the reaction between the metal NCs and the CL system including H2O2-nitrite [80], H2O2-fluorescein [81], luminol-H2O2 [82,83,84,85,86,87], KMnO4-rhodamine B [31,32], H2O2-rhodamine B [30], diperiodato- argentate-folic acid [88], KMnO4-rhodamine 6 G [89], H2O2-peroxymonocarbonate [90], luminol-NaIO4 [91], and K3Fe(CN)6-rhodamine 6 G [92]. In the presence of target, CL intensity is quenched or restored/enhanced. Up to now, there are about four ways to improve the catalytic activity of metal NCs in CL system: (1) charged metal NCs by modification; (2) bimetallic core NCs; (3) synergistic effect of nanomaterials; (4) Metal NCs encapsulated in uniform and well-ordered nano-porous structures. These strategies are also applied to improve the ECL efficiency of metal NCs.
3.2. Metal NCs as CL Luminophores
Compared with reports of the catalytic action of metal NCs, the investigation of metal NCs as luminophores of CL is rare. Li et al. designed a CL resonance energy transfer platform for sensitive and label-free detection of trypsin [35]. Bis(2,4,6-trichlorophenyl) oxalate (TCPO)-H2O2 acted as energy donor and BSA/Au NCs acted as energy acceptor. The BSA/Au NCs produced intense CL by accepting the energy from TCPO-H2O2 CL reaction. The CL intensity of BSA/Au NCs decreased when BSA was cleft by trypsin. As a consequence, a sensitive CL method for detection of trypsin was accomplished with a wide linear range from 0.01 μg mL−1 to 50.0 μg mL−1 with a detection limit of 9 ng mL−1. They continued to research direct CL of BSA/Au NCs with classic oxidants, such as KMnO4, N-bromosuccinimide, K3Fe(CN)6, H2O2 and Ce(IV) [34]. The highest CL signal was from acidic KMnO4-BSA/Au NCs CL reaction. The possible luminophore was the excited state Mn(II)*, originating from the reduction of KMnO4 with BSA/Au NCs. H2O2 can decreased the CL signal of acidic KMnO4- BSA/Au NCs system. Hence, the novel CL system was developed for the H2O2 determination with a linear range from 1.0 × 10−6 mol L−1 to 1.0 × 10−4 mol L−1. Cys/Cu NCs also played the role of reductant in the KMnO4- Cys/Cu NCs CL system [33]. Luminophore was the excited state Mn(II)*, originating from the reduction of KMnO4 with Cys/Cu NCs. Yuan et al. utilized hyperbranched polyethyleneimine (hPEI) as a template for the synthesis of hPEI/Ag NCs, which emitted CL by reacting with hydroxy radicals [36]. The emission originated from excited hPEI/Ag NCs. The molecular weight of hPEI affected the CL signal of hPEI/Ag NCs and 10 K hPEI/Ag NCs displayed the strongest CL signal. Tea polyphenols served as antioxidants consumed hydroxy radicals and resulted in a decrease in the CL signal. A sensitive detection method for tea polyphenols was developed based on this phenomenon. The excited state of Cys/Cu NCs also acted as a luminophore in the Ce(IV)-Cu NCs CL system [33].
3.3. Metal NCs as CL Quenchers
There are few reports on quenching CL of metal NCs in recent five years. Vahid et al. found that CdSe quantum dots (CdSe QDs) increased the CL intensity of H2O2-HCO3− system based on the CL resonance energy transfer (CRET) between the CL emitters and CdSe QDs and the catalytic activity of CdSe QDs [37]. BSA/Au NCs could prohibit the CRET system and turn off the CL emission. The CL signal was recovered because of the leaching effect of cyanide on Au NCs. This strategy resulted in a highly sensitive and reliable measurement of cyanide in environmental waters and biological samples.
4. Summary and Future Perspectives
In the past five years, tremendous literatures on the applications of metal NCs have been reported. In this presented review, we introduce recent research progress in the ECL and CL of metal NCs. Metal NCs with novel catalytic, electrical and optical properties can be obtained on various thiols, protein, DNA, polymer through chemical and electrochemical methods simply, rapidly and cheaply. On account of the advantages of metal NCs, such as small size, good biocompatibility, and luminosity, metal NCs gradually become a multipurpose tool promising for applications in sensing of DNAs and RNAs, metal ions, proteins and enzymes, small biomolecules and so on. This review focuses on the roles of metal NCs in CL and ECL fields, such as luminophores, catalysts, and quenchers. Many efforts have been devoted to enhance the ECL efficiency of metal NCs and the catalytic efficiency of metal NCs in CL system.
However, a great deal of challenges and difficulties remain in this exciting field of science. First, the chemical synthesis of metal NCs produces atomically precise but polydisperse clusters. In addition, the stability and dispersion of metal NCs has a certain influence on the luminous efficiency and catalytic efficiency. Therefore, it is urgent to explore new synthesis and purification methods to get metal NCs with single size, good stability and dispersion. Both Cu NCs and Ag NCs can improve their dispersion and control the size by designing DNA structures. DNA synthesis of Au NCs and other metal NCs has not been reported. Protein is one of the main protectants for the synthesis of metal NCs. Can peptides replace proteins in the synthesis of metal NCs? Can the size, dispersion and stability of metal NCs be improved if nanomaterials with special structures are introduced, such as MOF materials? These are all questions worth studying.
Second, although many kinds of metal NCs have been studied, such as Au, Ag, Pt, Cu, Ni, and some alloy NCs. It is still one of the directions to develop other non-noble metal NCs, alloy NCs or doped NCs to reduce costs and develop new properties. At present, the metal cores of alloy nanoclusters are mainly composed of two kinds of metals. There have been no clusters of three or more metallic elements. Doped elements are also very limited, electron-rich element doping may be one direction.
Third, the principles of metal NCs as luminescent agents, catalysts or quenchers are very complex. It is very important to study systematically the influence of metal nucleus, ligand, structure, solvent and charge on the properties of metal NCs. For example, whether the valence state-dependent ECL properties of the Au NCs can also be applied to other metal NCs? What about intramolecular electron transfer and aggregation-induced ECL? Only when one principle or strategy is verified on a variety of metal nanoclusters, its rationality can be effectively explained.
It is expected that with the recognition of the advantages of metal NCs by more researchers, emerging excellent work will be reported in the near future.
This entry is adapted from the peer-reviewed paper 10.3390/molecules25215208
