1. Alginate-Based Hydrogels and Scaffolds for Cancer Treatment
In recent decades, biodegradable alginate-based biomaterials have shown significant potential for the design of drug delivery systems (DDSs) for anticancer drugs
[1][2]. Alginate is an ideal material for designing and forming multifunctional drug delivery systems (DDSs) for cancer imaging and therapy due to its advantages such as biocompatibility, biodegradability, and convenient modification to different derivates with flexible properties, structure, functions, and applications
[3]. Moreover, it has the specific ability to form hydrogels with a high water content that makes them similar to native soft tissue. The alginate-based hybrid system of cisplatin-loaded alginate nanogel encapsulated in an injectable alginate hydrogel crosslinked with calcium ions for the treatment of ovarian cancer was developed
[4]. To form the cisplatin-loaded alginate nanogel, cisplatin (6 mg/mL) was mixed with an aqueous solution of alginate (2.0 mg/mL), heated at 95 °C, dialyzed, and lyophilized. Then, the cisplatin-loaded alginate nanogel was redispersed in a CaCl
2 solution and added to an alginate solution (50 mg/5 mL). The obtained system enabled high cisplatin loading capacity and the sustained release of cisplatin for over a week and showed significant in vivo antitumor activity. This therapy presented a modern approach to the treatment of advanced-stage ovarian cancer with KRAS mutations
[5]. A hybrid system based on alginate (100 mg/50 mL) and graphene oxide (GO) for DDS application was synthesized with calcium ions (0.1 wt%) as the crosslinker, followed by a freeze-drying procedure. The obtained DDS was evaluated for loading and controlled release of methotrexate (MTX), which is used as one of the chemotherapeutic agents for cancer therapy. The release study confirmed the pH- and electro-responsive release of MTX due to the pH-sensitive properties of alginate and the conductive ability of GO. It was observed that the cumulative release of methotrexate (MTX) was 14% at pH 7.40 and 9% at pH 6.00, but electrical stimuli increased the release of MTX to 29% and 37% at −0.2 V and −0.4 V, respectively. These findings represented a good starting point for the synthesis of smart DDSs based on polymers of natural origin
[6]. Some therapies involve the use of several therapeutic agents. Wu et al. investigated hydrogels with two co-assembling domains for structural reinforcement of the hydrogel and the controlled release of two drugs, cisplatin (CDDP) and irinotecan (IRN). It was observed that a fast release of CDDP occurred before the sustained release of IRN, suggesting that the release profile depended on the fraction ratio of IRN-loaded alginate nanoparticles in nanomaterials. Obtained in vitro release profiles of CDDP showed that 45% of the drug was released for 9 h, suggesting a fast release of CDDP where release rates of CDDP were unaffected by the amounts of INR-loaded alginate nanoparticles. On the other hand, the cumulative release of IRN was slower (11.3% for 9 h) and sustained (6 days 60.7%) with tunable properties by varying the fraction of IRN-loaded alginate nanoparticles in the nanosystem (from 0.4 wt% to 0.6 wt%). The obtained results provided a generalized strategy to realize the differential release of multiple active agents in dual therapy
[7]. In addition to the wide use of alginate in the preparation of single/combined DDSs, its application for the design of 3D scaffolds as tumor models is also significant
[7][8]. Its suitability for various biomedical applications implies that alginate has great potential in cancer therapy and research in this field. As can be seen throughout this research, alginate is not only an excellent candidate for the design of smart and stimuli-responsive DDSs for anticancer active agents but also an efficient 3D scaffolding material for cell cultures. Alginate-based hydrogels have been confirmed to improve traditional anticancer therapies and overcome existing disadvantages such as systemic toxicity and tumor resistance. Summarized data for alginate-based hydrogels and scaffolds for cancer treatment are presented in
Table 1.
2. Alginate-Based Hydrogels and Scaffolds as Antimicrobials
Antimicrobial materials are a very important class of materials in the biomedical area, especially now in the era when new types of microbes appear and pose a huge threat to the human race. Therefore, the choice of material types, in combination with bioactive agents, as well as production technologies are the focus of today’s modern scientific research. Some materials possess inherent antimicrobial properties, and some must “be enriched” with antimicrobial agents to achieve the final function. Alginate-based materials have a significant impact in the field of antimicrobial materials. This particularly applies to the treatment of skin function disorders. Wounds infected with a pathogenic microorganism usually become chronic and lead to the progression of life-threatening inflammatory diseases, and may also lead to cancer. Pathogenic microorganisms resistant to antimicrobial agents are an increasingly pressing problem that threatens the successful treatment of various types of infections
[9]. Heavy metals, copper, zinc, gold, and silver have been used for more than a millennium due to their known favorable antimicrobial properties. An advanced approach in terms of local delivery of heavy metals by choosing an appropriate drug release system is the answer to the threat of microbial infections
[9]. Alginate, as the basic polymer component in infection treatment systems, has proved to be the first choice. Hydrogel beads were obtained using alginate (0.5%
w/
v) with 1,3:2:4-di(4-acyl hydrazide)-benzylidene sorbitol (0.3%
w/
v), a component that self-assembles to form a gel
[10]. The antimicrobial properties of the hydrogel beads were acquired by the loading of silver ions and in situ reduction to form silver nanoparticles. Gels showed good antimicrobial properties against the drug-resistant bacterium
Pseudomonas aeruginosa (PA14) and Vancomycin-resistant
Enterococcus faecium (VAR). Calcium alginate greatly improves the thermal stability and mechanical properties of the gel. The combination of natural and synthetic materials allowed the hydrogel to have suitable mechanical features and the ability to manage the silver nanoparticle size and hydrogel shape. Silver nanoparticles, obtained similarly and stabilized by polyvinylpyrrolidone, were loaded in hydrogel made using alginate (2%
w/
v) and collagen (type I, 1%
w/
v)
[11]. Inclusion of alginate allowed for high water absorption capacity of the hydrogel, as well as improved mechanical properties. The charged surface of two natural polymers allowed for the formation of a polyelectrolyte complex. Hydrogel material displayed low cytotoxicity, with distinct antimicrobial properties against
Staphylococcus aureus and
Escherichia coli, dependent on the concentration of silver nanoparticles. Antimicrobial scaffolds were also created by combining negatively charged alginate (3%
w/
v) with positively charged polysaccharide chitosan (2.5%
w/
v) to form a polyelectrolyte complex network and loading it with silver nanoparticles
[12]. The cryogelation method afforded a porous thin-layer membrane with a large surface area that was tested for use as an air-filtration membrane to efficiently purify the air of microbes. Hydrogels showed inhibitory activity against
S. aureus and
E. coli. Novel antibacterial 3D-printed scaffolding materials obtained from a combination of alginate (5%
w/
v) and crystalline nanocellulose (CNC, 3%
w/
v) with incorporated silver nanoparticles have been reported
[13]. CNC is a biocompatible polymer of natural origin with a crystal structure and high surface area that is virtually non-toxic, making it a great component for the creation of biomaterials for medical applications. Combining it with alginate afforded the ability to greatly improve the mechanical properties of the hydrogel, with samples resisting breakage until a force of 3.58 N was applied. Hydrogels loaded with 10 and 100 µg/mL of silver nanoparticles displayed antimicrobial properties against
S. aureus and
P. aeruginosa, while not exhibiting any cytotoxic effects. Contrasted with the abovementioned hydrogels and scaffolds, materials based on alginate modified with a 3-(trimethoxy silyl)propyl)-octadecyl dimethyl ammonium chloride to produce quaternary ammonium salts have shown antimicrobial activity by themselves, even without the incorporation of silver
[14]. An aqueous solution of (3-(trimethoxysilyl)propyl)-octadecyl dimethyl ammonium chloride (0.02 M) was added to sodium alginate aqueous solution (4%
w/
v), and the resulting solution was added dropwise to a solution of calcium chloride (5%
w/
v). Loading the quaternary ammonium salt-based hydrogel with silver ions greatly increased its inhibitory activity against a wide variety of pathogenic microorganisms, including
Candida albicans, Methicillin-resistant
Staphylococcus aureus (MRSA),
S. aureus,
E. coli and
P. aeruginosa (ATCC
®10145 and ATCC
®27853), compared to conventional alginate-based hydrogels. The modified alginate structure also allowed the slow and constant release of silver ions, effectively reducing their toxicity while retaining antibacterial effects, making it a beneficial wound dressing material.
The antimicrobial properties of silver nanoparticles depend on the level of particle aggregation. Aggregated particles have a lower ratio of surface area to volume, leading to the non-optimal release of silver ions and reducing their potency to inhibit pathogenic microorganisms
[15]. Functionalized Ag nanoparticles with thioctic acid were prevented from aggregating to a large extent, and their antimicrobial properties were improved when incorporated into alginate hydrogel
[15]. An aqueous solution of sodium alginate (1.5%
v/
v) was used for the production of hydrogels. The addition of alginate enabled the formation of a stable hydrogel, with uniform distribution of silver nanoparticles. Modified silver particles were used to crosslink calcium alginate by ionic interactions between carboxyl functional groups and Ca
2+ ions, which allowed equal distribution of Ag particles in hydrogels.
Besides silver, other heavy metals with antimicrobial properties were also loaded in alginate hydrogels. Malagurski et al. managed to synthesize microbeads by crosslinking the alginate polymer mixture in a solution of copper(II) ions
[16]. By varying the concentration of the Cu
2+ ion solution in the range of 13.5–270 mM, added to the 1.9%
w/
v solution of sodium alginate, different sizes of microbeads were obtained. The Cu
2+ ion content controlled the release rate, with higher concentrations showing an initial “burst” effect, and lower Cu
2+ content leading to slower ion release from the hydrogel beads. Microbeads loaded with 100 µmol/g Cu
2+ showed rapid antibacterial effects against
E. coli and
S. aureus. The same authors also incorporated mineralized zinc (phosphate and carbonate) in alginate (1.9%
w/
v) by electrostatic extrusion, using zinc(II) ions gelling solution
[17]. Mineralized zinc was obtained by adding a 100 mM gelling solution of zinc nitrate to a saturated aqueous solution of sodium carbonate or sodium hydrogen phosphate. Hydrogels with mineralized zinc showed higher zinc(II) content and produced a sustained release of ions, as well as better stability in the physiological medium concerning non-mineralized samples. Both mineralized and non-mineralized samples showed strong antimicrobial effects against
S. aureus and
C. albicans, while concerning
E. coli, the effect depended on the type of sample. Three-dimensionally printed antimicrobial alginate/bacterial cellulose-based materials, with incorporated copper nanoparticles, active against
E. coli and
S. aureus strains were prepared
[18]. Alginate content was varied from 1 to 4%
w/
v. Two ionic crosslinking methods were tested, one using calcium ions with their subsequent replacement with copper ions by ion exchange and another crosslinking method with copper ions only. Copper nitrate aqueous solution (2.5 mg/mL) was treated with sodium borohydride solution to produce copper nanoparticles that were incorporated into alginate hydrogels. The ion exchange method yielded more-stable materials. Alginate is often used for 3D printing and to fabricate printable inks. New 3D printing inks based on antimicrobial alginate/bacterial-cellulose hydrogel structures with increased alginate content (4%
w/
v) showed improved printability compared to pure alginate hydrogels
[18], and the addition of copper provided antimicrobial effects against
E. coli and
S. aureus strains.
Antimicrobial cerium ion/chitosan crosslinked alginate (1%
w/
v) biopolymer films were produced using formulations of Ce
3+ and Ce
3+/chitosan (1%
w/
v) solutions as a crosslinker to compare results between films crosslinked using Ca
2+ and Ca
2+/chitosan solutions
[19]. Crosslinking of alginate was performed either by di- and trivalent cations or interactions with positively charged polyelectrolyte chitosan. Ce
3+ and Ce
3+/chitosan crosslinked films displayed better mechanical features (elastic modulus of 40.3 kPa and elongation on a break of 26.1%) compared to calcium-crosslinked alginate hydrogels (elastic modulus of 3.51 kPa and elongation on a break of 20.2%), and antibacterial properties. Furthermore, Ce
3+/chitosan film was shown to have the best antibacterial properties against
E. coli and
S. aureus among the samples and was used as a flexible, ultraviolet-protecting, antibacterial wound dressing. The injectable iron(III) ions-crosslinked alginate-hyaluronic acid hydrogel was produced as a hydrogel with typical shear-thinning properties
[20]. Alginate content was 3%
w/
v, and hyaluronic acid content was 0.75%
w/
v. Ionic crosslinking was performed by simply mixing the alginate-hyaluronic acid mixture with a Fe
3+/EDTA complex solution (0.1 mol/L Fe
3+ and 0.05 mol/L EDTA). The hydrogel was injected by application of shear stress, and it self-healed within seconds after the removal of shear, due to reversible and dynamic metal–ligand interactions between ferric ions and carboxyl groups of alginate-hyaluronic acid polymers. Besides good biocompatibility and degradability (complete degradation within 7 days), sustained ferric ions release from these hydrogels resulted in high antimicrobial activities against several types of bacteria, such as Gram-negative
E. coli and Gram-positive
S. aureus, as well as oral pathogenic bacteria
Streptococcus mutans and
Porphyromonas gingivalis.
Sodium alginate-based films were used to investigate the antimicrobial and antifungal activities of nine kinds of encapsulated essential oils
[21]. Films were formed by adding different volumes (0.1, 0.5, and 1.0 mL) of essential oils to an aqueous solution of sodium alginate (3%
w/
v) and glycerol (1%
v/
v). Dynamic mechanical analysis revealed that the addition of Igepal CO-520 surfactant improved the dispersion of the oils in the alginate matrix but resulted also in a lowering of Young’s modulus of the films (6 to 1 GPa), as well as their elongation rate at the brake, but to a lesser extent. By monitoring the release of oil, it was found that oils released from the films inhibited bacterial and fungal growth depending on the type and concentration of the oil. Almost all oils, except for chamomile blue, inhibit the growth of
C. albicans. Out of nine tested oils, only cinnamon, lemongrass and peppermint oil inhibited the growth of
E. coli. The antibacterial activity of lignin model dehydrogenated polymer (DHP) incorporated in alginate hydrogel was studied by using different bacterial strains and bacterial biofilms
[22]. DHP in DMSO (1%
w/
v) was added to an aqueous solution of sodium alginate (~2.1%
w/
v), and CaCl
2 (0.5%
w/
v) solution was used to crosslink the alginate. In addition to bacterial strains that were cultured in laboratory conditions, wild strains isolated from patients’ wounds were studied as well. The results showed that the lignin model dehydrogenated polymer was active against all tested Gram-positive and Gram-negative bacterial strains and had no cytotoxic effect on human Hep2c and HCjE cells. The best results were obtained against
L. monocytogenes,
P. aeruginosa,
S. typhimurium, and
S. aureus. Although alginate does not have antimicrobial activity, alginate hydrogel retained and immobilized bacteria, allowing for a reaction of the active substance with the bacteria
[23]. To investigate the angiogenic potential and antibacterial activity of heparin, three different concentrations (1, 5, and 10 µg/mL, marked A, B, and C, respectively) were loaded into commercially available alginate wound dressing biomaterials
[23]. The loaded hydrogel samples displayed a much higher swelling rate (1524, 1202, and 1279% for samples A, B, and C, respectively), compared to the non-loaded alginate sample (838%). The results indicated good absorption capability of hydrogels and increased angiogenic and antimicrobial ability caused by the addition of heparin. The best results for antibacterial activity against
E. coli with no cytotoxic effect were obtained with the highest heparin concentration of 10 µg/mL.
Sodium alginate and PEG-based hydrogels were prepared by Diels–Alder (DA) click chemistry between furyl-modified alginate (1.5%
w/
v aqueous solution) and PEG by two maleimide molecules
[24]. The hydrogels were subsequently chemically modified through grafting with cysteine (CYS)-terminated antimicrobial peptide HHC10–CYS in different concentrations (1, 2, 3, and 4 mg/mL) by thiol–ene reaction between the oxy-norbornene group and the thiol. The results of testing showed that hydrogels designed in this manner had good antimicrobial properties, with a sterilization rate against
E. coli > 98.5% for all samples, and were suitable for use as coatings for implantable medical devices. The healing efficacy of sodium alginate (10%
w/
v) hydrogel was advanced by coupling it with different concentrations of honey (2–10%) and performing dual ionic, with calcium cations, and covalent crosslinking with maleic anhydride
[25]. The dually crosslinked sodium alginate hydrogels with 4% honey concentration produced a material with appropriate stiffness (2.32 µN/nm), swelling behavior (highest swelling index of 0.6), and controlled degradation (87.36% after 12 days), providing wound dressing material with the best environment necessary to improve cell growth and proliferation. This wound dressing also had good antimicrobial potential against Methicillin-resistant
S. aureus and
E. coli. Alginate hydrogels, prepared from 1%
w/
v aqueous solution of sodium alginate crosslinked by calcium chloride, were loaded with neomycin or propolis by direct blending or by adding neomycin (0.01% neomycin sulfate solution) or propolis-loaded alginate microparticles prepared by the extrusion dripping method to alginate hydrogels
[26]. The resulting films were tested for physical and antimicrobial penetration. The loading of neomycin and propolis, in both types of films, provided the samples with high swelling degrees of up to 100%. The films also provided sufficient antibacterial action to stop the penetration of microorganisms in a wound, which is essential for absorbent wound dressing materials. Summarized data for alginate-based antimicrobial materials are presented in
Table 2.
3. Alginate, Gelatin, and 2-Hydroxyethyl Methacrylate Based Hydrogel Scaffolds for Selected Areas of Biomedical Applications
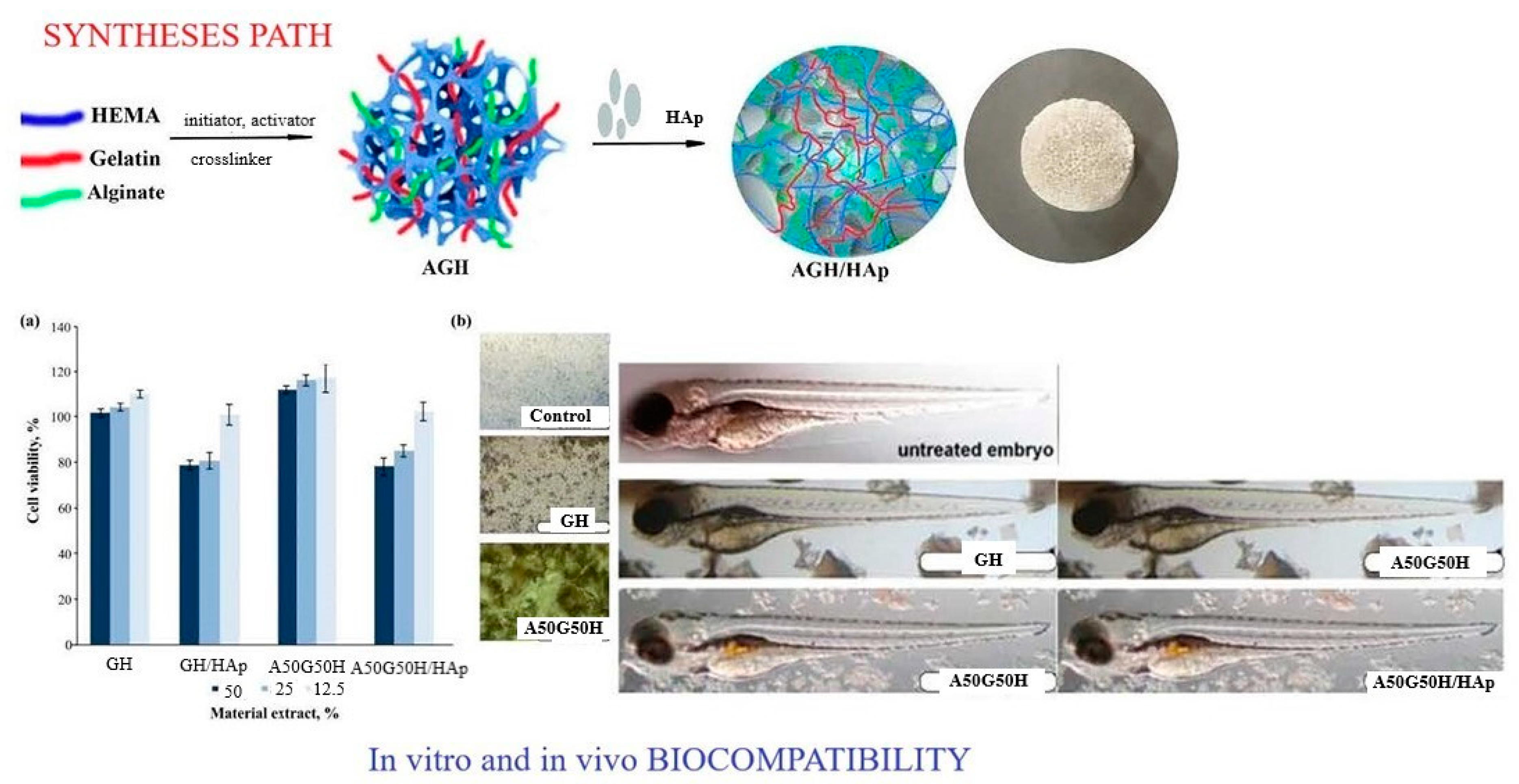
A multifunctional polymeric system was designed using alginate combined with gelatin, 2-hydroxyethyl methacrylate, and bioactive components and agents so that hydrogel scaffolds with specific properties suitable for biomedical applications were obtained. Biocompatible, porous, and degradable hydrogel scaffolds made of alginate/gelatin/2-hydroxyethyl methacrylate (AGH), embedded with inorganic agent apatite (HAp), were made using the cryogelation method (
Scheme 1). A balance was achieved between the biological and mechanical properties of the created hydrogel scaffolds for advanced tissue regeneration applications. The component weight ratio was gelatin/HEMA = 0.2/0.8 and alginate/gelatin/HEMA = 0.1/0.1/0.8. The sodium salt of alginic acid from brown algae was used for all syntheses. It has been shown that the loading of linear alginate chains affects the mechanical properties in terms of increasing modulus and decreasing porosity. All hydrogel scaffolds showed a completely hydrophilic character, which made them suitable biomaterials for scaffolds with improved adhesion, proliferation, and differentiation of cells in the tissue regeneration process. An in vitro degradation study was conducted for 3 months, and the results showed that alginate affected the degradability of the scaffolds due to its hydrophilic character, which promotes higher degradability. An in vitro biocompatibility test on a fibroblast cell line and an in vivo study on a zebrafish model confirmed that the obtained scaffolds had satisfactory biocompatibility and that alginate contributed to better biocompatibility. These specific properties indicated that the created alginate/gelatin/2-hydroxyethyl methacrylate/HAp hydrogel scaffolds possessed advanced properties for biomedical applications
[27].
Scheme 1. Syntheses path for preparation AGH/HAp scaffolds; in vitro (fibroblast cell line (A)) and in vivo (zebrafish (B)) biocompatibility of alginate/gelatin/2-hydroxyethyl methacrylate/apatite scaffolds.
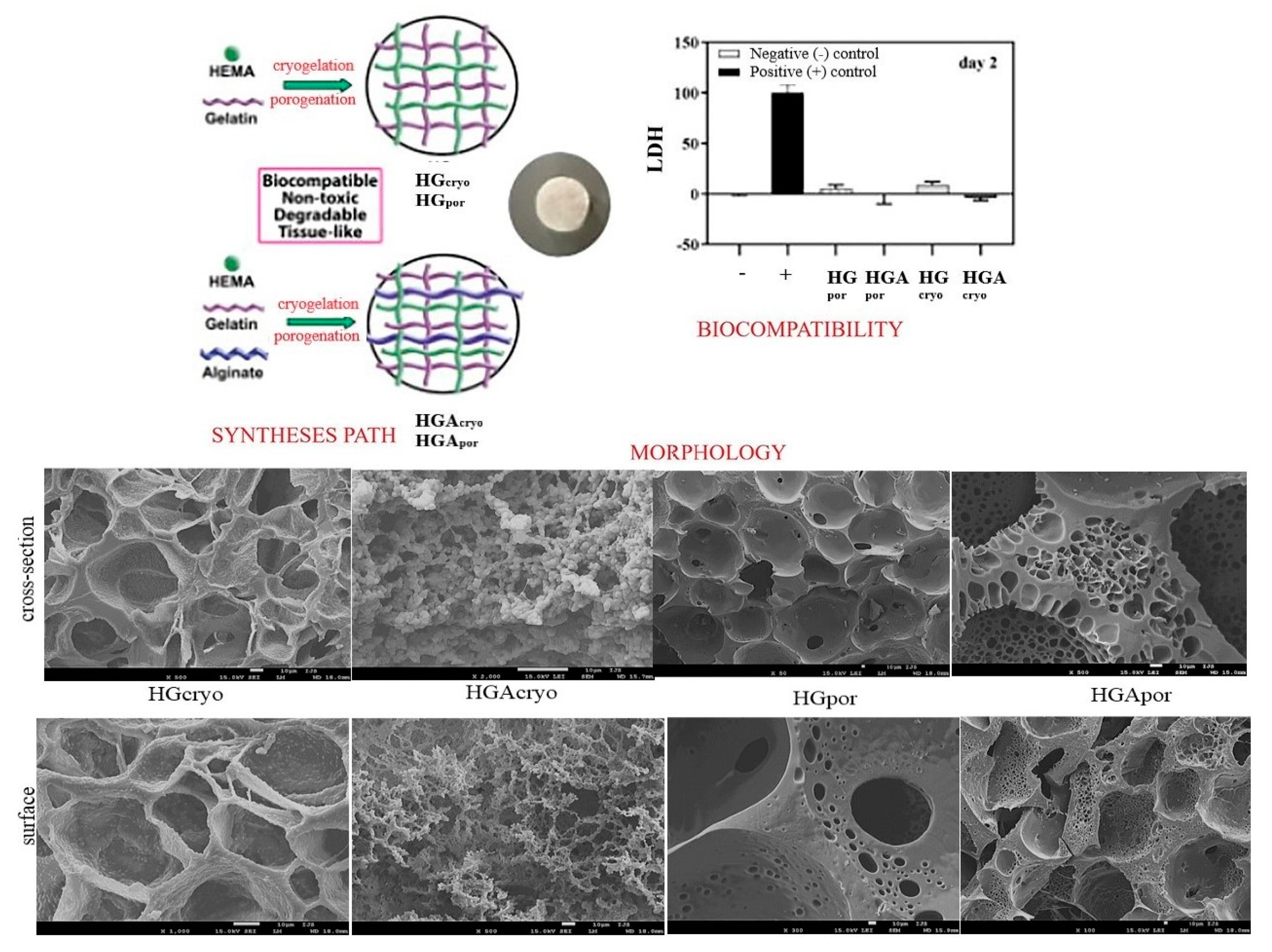
Bioactive 2-hydroxyethyl methacrylate (HEMA)/gelatin (G)/alginate (A) biomaterials for scaffolds in combination with inorganic agent apatite (HAp) were produced by cryogelation (−18 °C) and porogenation (using porogen agent) methods (
Scheme 2). The ratio of alginate to gelatin as the weight ratio was A/G (50/50 for cryosamples and porogen samples). The sodium salt of alginic acid from brown algae was used for all syntheses. Our scientific concept was based on the creation of a “replica” of the native extracellular matrix through the design of interpenetrating networks of polymeric hydrogels. The key influence of the method of preparation and introduction of linear alginate as an interpenetrant into the HEMA/gelatin polymeric networks on the properties of the obtained hydrogel scaffold was confirmed. The morphological patterns were satisfactory interconnected pore structures depending on the interpenetrating linear alginate chains and the preparation method. Porosity values ranged from 66.38–84.25% and indicated the dominant influence of linear alginate chains, as well as scaffold preparation methods. Mechanical properties expressed by Young’s modulus were in the range of 0.99–9.75 MPa. Alginate was found to improve the mechanical features of the cryogelated scaffold and decrease the porogenated scaffold. Porosity values ranged from 66.38–84.25%. The mechanical properties given using Young’s modulus were in the range of 0.99–9.75 MPa. In vitro degradation was studied over a time interval of 16 weeks and showed a weight loss percentage of 1.59–5.04%. This behavior during degradation is explained by the higher porosity of the cryosamples and the hydrophilicity of alginate, which allows faster and easier penetration of water inside the scaffolds and a higher degree of degradation. The cell viability test showed excellent biocompatible behavior of the scaffolds. Alginate as a component improved biocompatibility. Cryogelled scaffolds have been shown to possess superior biofunctionality necessary for the tissue regeneration process—full hydrophilicity, degradability, appropriate mechanical properties, and favorable biocompatibility. The introduction of the inorganic agent apatite into the cryogelated scaffolds showed a favorable cell adhesion capacity and good biocompatible and non-toxic properties. All synthesized scaffolds performed equally in terms of metabolic activity and osteoconductivity. The study showed that cryogelated scaffolds with/without HAp were a breakthrough for improving osteoconductivity and intensifying hard tissue regeneration
[28].
Scheme 2. Syntheses path, biocompatibility, and morphological features of IPN and semi–IPN 2-hydroxyethyl methacrylate/gelatin intertwined with alginate and doped with apatite scaffolds (the bar is 10 μm).
Embedding of iron(III) oxide in 2-hydroxyethyl methacrylate/gelatin/alginate hydrogel scaffolds resulted in iron(III) oxide/2-hydroxyethyl methacrylate/gelatin/alginate hybrid hydrogel scaffolds (multicomponent Fe/H/G/A biomaterials). A multifunctional, biocompatible, porous, and degradable hydrogel scaffold platform based on 2-hydroxyethyl methacrylate, gelatin, and alginate integrated with iron(III) oxide was obtained using a simple and effective, modified porogenation method. To achieve a balance between the biological and mechanical properties of Fe/H/G/A hydrogels and to satisfy their final biomedical application as scaffolds and efficient drug delivery systems, the composition of the hydrogels was varied. The proportion of alginate was shown concerning gelatin as a weight ratio A/G (100/0, 50/50, and 70/30). Alginic acid sodium salt from brown algae was used for all syntheses. Fe/H/G/A hydrogels showed pH sensitivity with maximal swelling at pHs 2.20 and 7.40 at 37 °C as well as tunable swelling capacity by simply controlling their composition. The sample containing the highest content of alginate showed the highest swelling degree. With an increase in the alginate content in these samples, there was a decrease in porosity, because the linear alginate chains rearranged themselves within the HG network and filled the free space (porosity range of 64.97–72.38%). All Fe/H/G/A hybrids manifested fully hydrophilic character, which made them convenient for scaffolding biomaterials to support population, adhesion, and differentiation of cells. In vitro degradation studies for 3 and 6 months showed that samples containing higher alginate content had a higher level of degradability. The samples degraded up to 23.90% after 3 months, while after 6 months, they degraded up to 45.69%. In vitro biocompatibility assays confirmed that hybrid scaffolds with higher alginate content possessed excellent biocompatible behavior. The hydrophobic drug curcumin was efficiently loaded into the Fe/H/G/A hybrids, with an efficiency of over 95%. Fe/H/G/A hybrids, as controlled curcumin release platform with high release ability for hydrophobic drugs, indicated that the more alginate these hybrids contained, the better the release performances were achieved. The specific properties of the obtained iron(III) oxide/2-hydroxyethyl methacrylate/gelatin/alginate hybrids developed in this research exhibited favorable features for biomedical applications, as well as for the design of a controlled drug release platform for hydrophobic drugs
[29].
Multifunctional graphene oxide/2-hydroxyethyl methacrylate/gelatin/alginate hydrogel scaffolds (GO/H/G/A) as scaffolding biomaterials were set as suitable controlled drug release systems. The hydrogels were prepared using the modified porogenation method. The ratio of alginate to gelatin as a weight ratio was A/G (100/0, 50/50, and 70/30). Alginic acid sodium salt from brown algae was used for syntheses. The engineered hydrogel scaffolds showed satisfying swelling capacity, full hydrophilicity, porosity (range of 56 to 76%), mechanical properties (Young’s modulus values from 1.69 to 4.78 MPa), and in vitro degradation up to 40% for 6 months. Alginate was a key component that influenced all of the tested properties, and with an increase in content, the tested properties were improved. The entrapment efficiency of curcumin (therapeutic bioactive agent) was above 99%. The controlled release process indicated excellent curcumin release performances for the obtained scaffolding systems, emphasizing that the sample with the highest alginate content showed the best release performances. An in vitro cytotoxicity assay on healthy human fibroblasts revealed non-toxic and favorable biocompatibility. The unique performance of GO/PHEMA/G/A hydrogel scaffolds indicated applications in various medical fields, as well as favorable controlled drug release systems
[30].
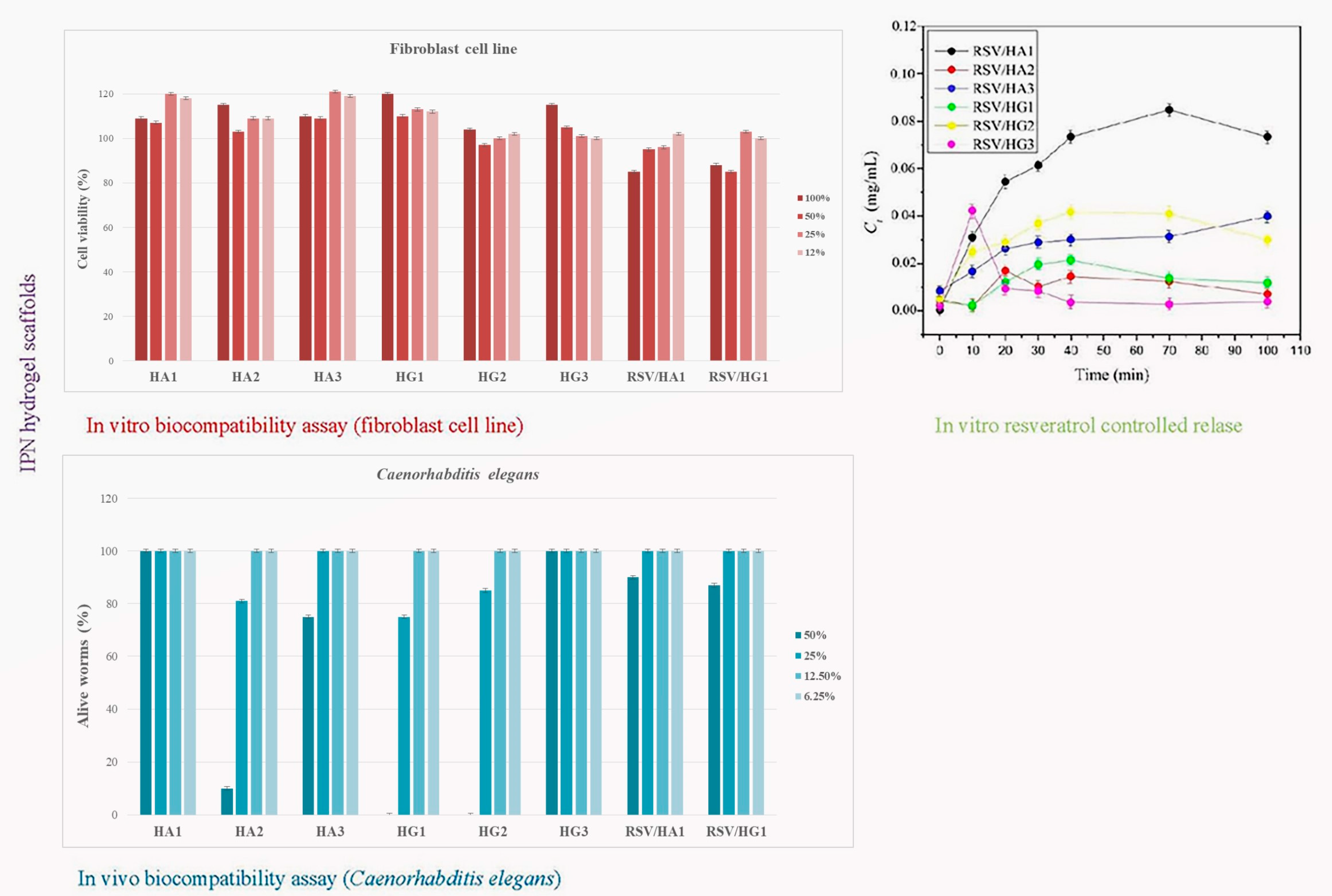
Interpenetrating hydrogel networks (IPN) of 2-hydroxyethyl methacrylate/alginate (HA) and 2-hydroxyethyl methacrylate/gelatin (HG) hydrogel scaffolds were obtained to design biocompatible and controlled resveratrol release platform (
Scheme 3). Interpenetrating hydrogel networks consisting of 2-hydroxyethyl methacrylate (H), alginate (A), and gelatin (G) were synthesized using cryogelation at −18 °C for 24 h (ratio H/A and H/G = 0.8/0.2). Three combinations with two types of crosslinkers were used (N-ethyl-N′-(3-dimethyl aminopropyl)carbodiimide hydrochloride-EDC and N-hydroxysuccinimide-NHS). Multifunctional, bioactive scaffolding biomaterials possess advantageous absorption ability, specific morphological patterns with interconnected pores, full hydrophilicity, a porosity range of 62.36 to 85.20%, and mechanical properties expressed by Young’s modulus from 1.40 to 7.50 MPa, dependent on the scaffold’s composition and the used crosslinkers and their concentrations. In vitro and in vivo assays performed on human fibroblast cell line and the worm
Caenorhabditis elegans exhibited favorable biocompatible features (cell viability and nematodotoxicity) of prepared IPN HA and HG hydrogel scaffolds that depended on the scaffold’s composition and the used crosslinkers and their concentrations. The release parameters of resveratrol/HA and resveratrol/HG systems were determined and suitable release models are defined. The most favorable properties for biomedical applications of these two series of IPNs were shown by samples containing alginate and EDC crosslinker. According to the authentic features, scaffolding platforms based on IPN HA and HG series were effective controlled resveratrol release systems
[31].
Scheme 3. In vitro and in vivo biocompatible and controlled resveratrol release performances of IPN HA and HG scaffold series.
This research opus for hydrogel scaffolds based on alginate, gelatin, HEMA, and bioactive agents has shown that the introduction of alginate as a component in these polymeric multi-systems, which contained several other polymeric components and bioactive agents, had an advanced, improved effect on most of the properties that were examined and most important for biomedical applications (Table 3). It should be noted that there are different types of alginates as well as newer and more sophisticated methods of synthesis and characterization. All research efforts are aimed at creating scaffolds that are closer in properties and functions to natural tissues. The main goal is to create bionic tissues and organs that perform nearly as well as natural tissues and organs and provide a longer and better quality of life for the human population.
Table 3. Summarized data for alginate, gelatin, and HEMA-based hydrogel scaffolds with bioactive agents for biomedical applications.
This entry is adapted from the peer-reviewed paper 10.3390/md21030177