Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Retroperitoneal sarcomas (RPSs) are locally aggressive tumors that can compromise major vessels of the retroperitoneum including the inferior vena cava, aorta, or main tributary vessels. Vascular involvement can be secondary to the tumor’s infiltrating growth pattern or primary vascular origin.
- retroperitoneum
- sarcoma
- vascular reconstruction
1. Vascular Involvement
When defining resectability, it is important to characterize the extent of vascular resection and type of reconstruction required. The organs included in the multi-visceral resection will be determined by predicted tumor infiltration or by involvement of the primary vasculature of organs with end circulation. Major blood vessels in the retroperitoneum such as the vena cava, iliac vessels, aorta, or any major tributary can be involved at any segment in an RPS. Vascular involvement can be partial or circumferential and can be associated with or without vascular flow obstruction.
Primary vascular sarcomas of the retroperitoneum can require complex vascular reconstructions, albeit with a less extensive multi-visceral resection since they normally don’t infiltrate other surrounding organs. Primary malignant vascular sarcomas of the retroperitoneum include mainly LMSs and angiosarcomas; RP-LMSs arise from the smooth muscle layer of vasculature of the retroperitoneum with a significant proportion that develop from large blood vessels such as the vena cava, iliac, or renal veins. Caval LMSs have a slight gender predominance in women and can develop at any segment of the vena cava, from the iliac vein confluence to the right atrium of the heart. Caval LMSs can be defined following Vollmann’s classification depending on level of origin within the vena cava as type 1 (originating between the confluence of main iliac veins and below the level of renal veins), type 2 (originating at the level of renal veins), type 3 (originating above renal veins and below major hepatic veins), and type 4 (at the level of the major hepatic veins and right atrium) (Figure 1) [1][2].
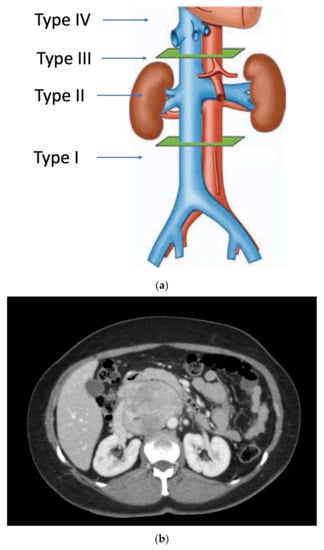
Figure 1. (a) Vollmann’s IVC-LMS classification; (b) type 2 IVC-LMS.
Caval LMSs can present with symptoms secondary to vascular occlusion such as vague abdominal pain, lower extremity edema, deep venous thrombosis, or pulmonary embolism. They are biologically aggressive, with a predominant metastatic pattern of failure after curative-intent resection [3][4]. Certain studies have described a worse survival outcome of vascular originating LMS compared to other originating sites [5]. Most reports on caval LMSs are based on small patient series that do not allow for strong evidence-based conclusions; nonetheless, there is a consensus that surgery offers the best chance for prolonged survival in patients with this rare tumor [6][7].
RP-LMSs can also develop from major arterial structures such as the aorta, superior mesenteric artery, or the celiac trunk (Figure 2). Within aortic primary sarcomas, angiosarcomas represent another subset of ultrarare but aggressive tumors with poor outcomes and 5-year survival rates quoted at 8%, portraying a dismal prognosis mostly secondary to metastatic failure or tumor-related complications such as tumor embolization or ostial occlusion, resulting in mesenteric infarction [8].
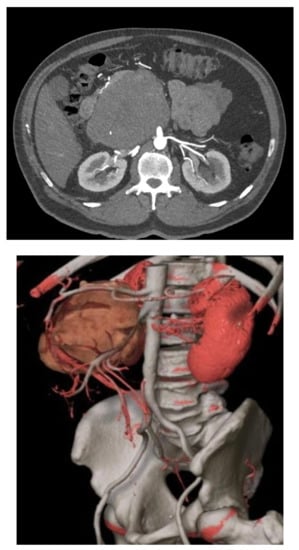
Figure 2. Mesenteric artery LMS: angio-CT and 3D reconstruction.
2. Surgical Planning
Surgical planning for complex vascular resections is critical and mandates adequate preoperative imaging. The rational for surgical approach varies depending on the tumor histology type. RP-LPSs require a more extensive multi-visceral approach since the limit between tumor tissue and normal retroperitoneal fat cannot be accurately predicted on preoperative imaging or intraoperative exploration. This compartmental approach should include the removal of all the ipsilateral fat, colon, kidney, and psoas fascia, plus other infiltrated organs such as the pancreatic tail and major vessels. Primary RP vascular sarcomas (LMSs/angiosarcomas), on the contrary, do not require such an extensive multi-visceral approach as the tumor boundaries are better predicted on preoperative imaging.
Currently, contrast-enhanced multiphase abdominal computed tomography (CT) with arterial and venous phases is preferred. Tumors arising from large vessels may be intraluminal, extraluminal, or a combination of both; therefore, contrast-enhanced cross-sectional imaging is necessary to provide a detailed evaluation of the size and extent of the tumor, as well as other characteristics such as internal tumor hemorrhage, necrosis, or cystic changes [9].
Spiral CT angiography with 3D reconstruction has become an important tool for surgical planning in tumors originating or infiltrating arterial structures such as the aorta, superior mesenteric artery, celiac trunk, or iliac arteries. The angiography can help to better predict the vascular tissue infiltration versus vascular abutment, whereas the 3D reconstruction can help to determine surrounding organ involvement and accurately plan the type of reconstruction required (Figure 2).
Abdominal magnetic resonance imaging (MRI) can be employed when a CT scan with intravenous contrast cannot be obtained. It may also help to better determine the presence of vascular collaterals when analyzing to reconstruct or simply ligate a major retroperitoneal vein following resection. The use of positron emission tomography CT (PET-CT) is not considered the standard of care for disease staging or for surgical planning in RPSs. However, several studies have explored the complementary role of PET-CT in the grading of STS [9].
3. Vascular Approach and Types of Reconstruction
Vascular resections can be partial or complete. The type of reconstruction, if any, will be determined by the type of vessel, extent of the tumor infiltration, and patency of vessel at the time of resection. Venous resections can be partial (side-wall resection) as they have greater compliance than arteries due to their thinner smooth muscle layer. Partial venous resections can be performed with a vascular stapler or can be performed sharply with subsequent closure of venotomy with primarily suture venorraphy or a patch using a biologic (e.g., bovine pericardium) or synthetic graft to avoid critical venous narrowing after the tumor has been resected (Figure 3).
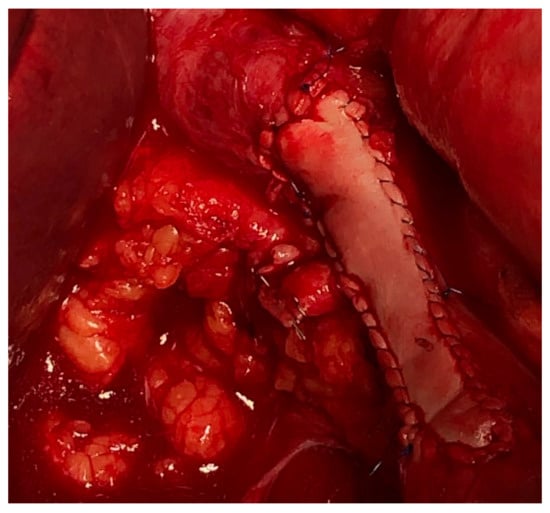
Figure 3. IVC partial resection with patch repair.
Arterial resection typically requires a formal segmental resection. Depending on the length of the tumor involvement, arterial repair my require an end-to-end anastomosis or an interposition bypass graft. There are many grafts options available, ranging from homologous venous grafts (e.g., saphenous, left renal, femoral, or jugular vein) to synthetic (e.g., Dacron, PTFE, and Goretex) or cadaveric grafts. The choice of graft depends on various factors: (i) clean vs. clean–contaminated case in the presence of concomitant visceral resection, (ii) availability and access to the various types of synthetic and cadaveric grafts, and (iii) surgeon preference.
3.1. Vena Cava Reconstruction
Reconstruction of the vena cava will vary, depending on the tumor location, extent of venous wall involvement, and the presence of chronic obstruction with established venous collateral outflow.
As aforementioned, tumor involvement of the IVC can be defined according to Vollman’s classification (Types 1–4) (Figure 1).
Others have simplified this classification in only three portions: upper portion (Level 1), extending from the entry of the hepatic veins up to the right atrium; middle portion (Level 2), extending from the renal veins to the hepatic veins; lower portion (Level 3), extending from the confluence of the iliac veins to the entry of the renal veins [10]. However, the distinction between the subtype involving the hepatocaval confluence to all other retrohepatic IVC-LMSs is important, since IVC-LMSs involving segment IV are generally considered nonresectable tumors.
Suspected vena cava involvement on preoperative imaging for an RP-LPS is assessed at the time of surgical exploration. Most retroperitoneal WD-LPSs and many DD-LPSs simply displace but do not infiltrate the caval wall [11]; thus, they can be safely dissected from the vena cava adventia without need of a formal caval resection, whether partial or complete. On the contrary, caval LMSs routinely require venous resection, partial or complete; thus, a primary venorrhaphy, patch repair, or complete interposition graft may be required. Additionally, primary renal or right gonadal vein LMS can involve the caval lumen and necessitate caval repair.
3.2. Iliac Vessels
Iliac vessels, including the vein and artery, can be compromised either by retroperitoneal sarcomas projecting into the pelvis or by pelvic sarcomas. The most common histologic subtypes are RP-LMSs and RP-LPSs, as well as others more frequently found in the pelvis including solitary fibrous tumors, synovial sarcoma, MPNST, or Ewing’s sarcoma [1][12]. Iliac vessels (vein and artery) are anatomically in closer proximity; therefore, it is not uncommon that both vessels may be involved in the tumor mass, requiring a combined artery and vein reconstruction.
The iliac artery reconstruction is fashioned similarly with synthetic or biologic graft material. The internal iliac artery can be ligated and not reimplanted to the graft. If the graft crosses the inguinal ligament to be anastomosed to the femoral artery, consideration of a musculocutaneous or myofascial flap to cover and protect the synthetic graft from superficial exposure must be undertaken [13]. When iliac vessels are reconstructed with a concomitant visceral resection, consideration for an extra-anatomical bypass (femoral artery to femoral artery) can be performed if there is an issue with availability and access to biologic grafts or the surgeon believes the risk of graft infection is too high to consider using a synthetic graft. Another possibility in the absence of banked tissue is to harvest the contralateral femoral artery or vein, reconstructing it with synthetic graft, and using the autologous graft to replace the resected iliac artery.
Management of the iliac vein follows similar dogmas as caval resections: patch repair, interposition graft, or ligation. The iliac vein is divided into two segments to plan reconstruction: segment I is defined between the origin of the external iliac vein and the internal iliac/hypogastric vein to the ilioinguinal ligament; segment II is defined by the common iliac vein per se. Reconstruction of the common or external iliac veins can be fashioned with an autologous left renal vein, contralateral femoral vein, cryopreserved cadaveric graft, or PTFE graft. Patency after reconstruction is lower compared to IVC reconstruction, favoring the use of PTFE with a 71% long-term patency [12] (Figure 4). When iliac vein resection includes the internal iliac inflow or hypogastric vein, this vein can simply be ligated.
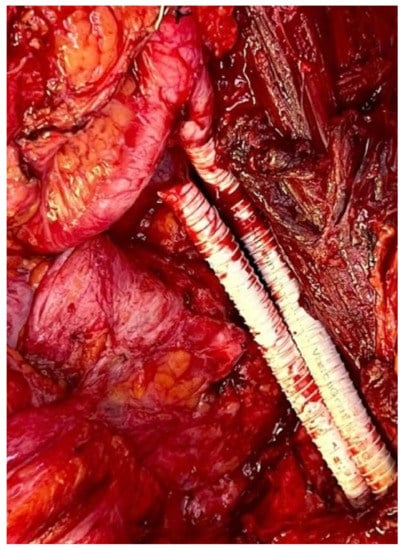
Figure 4. Iliac artery and vein reconstruction after DD-LPS resection.
3.3. Aortic Replacement
Primary sarcomas of the aorta can be categorized by histology and according to where they appear to arise (i.e., aortic media or intima), among which intimal sarcoma types are the most common [14]. In the context of RPSs with major vascular involvement, the aorta is generally infiltrated by high-grade nonvascular sarcomas such as DD-LPSs.
Oncovascular replacement of the abdominal aorta is generally performed at the infrarenal aorta level. The circumferential tumor involvement of the suprarenal aorta implies a very high-risk procedure due to the debranching and reimplantation of renal and mesenteric arteries (e.g., superior mesenteric artery and celiac trunk). Considering that this aortic reconstruction could include a multi-visceral reconstruction for primary/recurrent high-grade nonvascular RPSs (DD-LPSs), the perioperative mortality most certainly would surpass the oncologic survival benefit; therefore, circumferential suprarenal aortic tumor involvement for nonvascular primary disease is generally considered nonresectable.
In primary aortic STSs, such as angiosarcomas, resection and reconstruction of the aorta may not require a multi-visceral resection. Therefore, the risk of suprarenal aortic reconstruction may be undertaken considering it is the only therapeutic option for a primary aggressive tumor.
This entry is adapted from the peer-reviewed paper 10.3390/curroncol30030266
References
- Fiore, M.; Colombo, C.; Locati, P.; Berselli, M.; Radaelli, S.; Morosi, C.; Casali, P.G.; Gronchi, A. Surgical technique, morbidity, and outcome of primary retroperitoneal sarcoma involving inferior vena cava. Ann. Surg. Oncol. 2012, 19, 511–518.
- Ridwelski, K.R.S.; Meyer, F.; Buhtz, P.; Burger, T.; Lippert, H. Primary sarcoma of the inferior vena cava: Review of diagnosis, treatment, and outcomes in a case series. Int. Surg. 2001, 86, 140–190.
- Gronchi, A.; Strauss, D.C.; Miceli, R.; Bonvalot, S.; Swallow, C.J.; Hohenberger, P.; Van Coevorden, F.; Rutkowski, P.; Callegaro, D.; Hayes, A.J.; et al. Variability in Patterns of Recurrence After Resection of Primary Retroperitoneal Sarcoma (RPS): A Report on 1007 Patients From the Multi-institutional Collaborative RPS Working Group. Ann. Surg. 2016, 263, 1002–1009.
- Gladdy, R.A.; Qin, L.X.; Moraco, N.; Agaram, N.P.; Brennan, M.F.; Singer, S. Predictors of survival and recurrence in primary leiomyosarcoma. Ann. Surg. Oncol. 2013, 20, 1851–1857.
- Italiano, A.; Toulmonde, M.; Stoeckle, E.; Kind, M.; Kantor, G.; Coindre, J.M.; Bui, B. Clinical outcome of leiomyosarcomas of vascular origin: Comparison with leiomyosarcomas of other origin. Ann. Oncol. 2010, 21, 1915–1921.
- Cananzi, F.C.; Mussi, C.; Bordoni, M.G.; Marrari, A.; De Sanctis, R.; Colombo, P.; Quagliuolo, V. Role of surgery in the multimodal treatment of primary and recurrent leiomyosarcoma of the inferior vena cava. J. Surg. Oncol. 2016, 114, 44–49.
- Hollenbeck, S.T.; Grobmyer, S.R.; Kent, K.C.; Brennan, M.F. Surgical treatment and outcomes of patients with primary inferior vena cava leiomyosarcoma. J. Am. Coll. Surg. 2003, 197, 575–579.
- Fatima, J.; Duncan, A.A.; Maleszewski, J.J.; Kalra, M.; Oderich, G.S.; Gloviczki, P.; Suri, R.M.; Bower, T.C. Primary angiosarcoma of the aorta, great vessels, and the heart. J. Vasc. Surg. 2013, 57, 756–764.
- Devaud, N.; Vornicova, O.; Abdul Razak, A.R.; Khalili, K.; Demicco, E.G.; Mitric, C.; Bernardini, M.Q.; Gladdy, R.A. Leiomyosarcoma: Current Clinical Management and Future Horizons. Surg. Oncol. Clin. N. Am. 2022, 31, 527–546.
- Kulaylat, M.N.; Karakousis, C.P.; Doerr, R.J.; Karamanoukian, H.L.; O’Brien, J.; Peer, R. Leiomyosarcoma of the inferior vena cava: A clinicopathologic review and report of three cases. J. Surg. Oncol. 1997, 65, 205–217.
- Fairweather, M.; Wang, J.; Jo, V.Y.; Baldini, E.H.; Bertagnolli, M.M.; Raut, C.P. Surgical Management of Primary Retroperitoneal Sarcomas: Rationale for Selective Organ Resection. Ann. Surg. Oncol. 2018, 25, 98–106.
- Ferraris, M.; Callegaro, D.; Barretta, F.; Fiore, M.; Radaelli, S.; Stacchiotti, S.; Miceli, R.; Socrate, A.M.; Locati, P.; Gronchi, A. Outcome of iliocaval resection and reconstruction for retroperitoneal sarcoma. J. Vasc. Surg. Venous. Lymphat. Disord. 2019, 7, 547–556.
- Schwarzbach, M.H.; Hormann, Y.; Hinz, U.; Leowardi, C.; Bockler, D.; Mechtersheimer, G.; Friess, H.; Buchler, M.W.; Allenberg, J.R. Clinical results of surgery for retroperitoneal sarcoma with major blood vessel involvement. J. Vasc. Surg. 2006, 44, 46–55.
- Thalheimer, A.; Fein, M.; Geissinger, E.; Franke, S. Intimal angiosarcoma of the aorta: Report of a case and review of the literature. J. Vasc. Surg. 2004, 40, 548–553.
This entry is offline, you can click here to edit this entry!
