Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Agriculture, Dairy & Animal Science
Soil contamination with organic contaminants and various heavy metals has become a global environmental concern. Biochar application for the remediation of polluted soils may render a novel solution to soil contamination issues.
- biochar
- bio-availability
- heavy metals
- pyrolysis
- soil contamination
- toxicity
1. Biochar Application to Polluted Soils
Biochar has the ability to treat the polluted soil with organic and inorganic elements, decrease the soil nutrient leaching loss, and amend the physiochemical attributes of soil.
Improving the Soil Traits
The soil improvement under biochar application is mostly reflected in the amendment of soil organic matter (SOM), the improvement of nutrient dynamics and utilization rate, and the amendment of acidic soil and soil erosion [14]. Moreover, biochar traits, such as higher surface area and porosity, enhance the water-holding capacity (WHC), the soil porosity and soil capacity, and the porous structure of biochar, which make it a better habitat for the soil microbial population (Figure 1). Biochar application can efficiently improve the structure of soil, decrease the moisture content loss because of structure runoff and filtration, and enhance soil-available water [17]. Montagnoli et al. [18] reported that the biochar’s higher porosity has strong water retention ability, and the slow discharge of water contents from biochars can significantly enhance the water conservancy properties of degraded soil. Biochar incorporation into the soil can enhance the pH levels, possibly due to the biochar’s higher base cation composition, such as Na+, K+, Mg2+, and Ca2+. Biochar ash content is comprised of carbonates and hydroxides, and these substances’ dissolution expedites the soil pH increase of enhancement [19]. The negatively charged surface functional groups of biochar can greatly adsorb cations and support an increase in the soil’s CEC [20]. Oni et al. reported that the composition of feedstocks determines the biochar CEC during pyrolysis mechanism [21]. Jain et al. [22] reported the immobilization of metal ions in the soil via substituting cations on the biochar, and these cations enter into the soil and enhance the pH. Therefore, the elevation of soil pH and CEC induced through biochar might be due to the decline of metal ions bio-availability. Therefore, biochar is frequently applied for soil reclamation polluted by cationic-trace components [22]. Biochar contains essential nutrients, such as Ca, Mg, K, P, and N. These elements are necessary for plant growth and development, and the release of these nutrients stimulates or accelerates the growth of plants [23]. Biochars produced from different feedstocks also varied in nutrient substances, for example, biochar derived from grass seed had a higher amount of P, whereas biochars derived from wood contained more Mg and Ca [24]. Moreover, the biochar’s strong WHC can decrease nutrient leaching, modify the nutrient dynamics, stimulate the root nodule, and accelerate the plant growth and immobilization of N [25]. Furthermore, the minimum dose of biochar needed to maintain plant growth also varies because of the diversity in the heavy metal and nutrient concentration of polluted soils [26]. The higher amount of organic and inorganic pollutants in soils causes a disturbance in soil enzyme activity or functionalities, and the microbial population may be seriously damaged [27]. Applying biochar can improve the habitats of the microbial community, by influencing the structure, diversity, microorganism’s activity, and nutrient availability [28]. Torabian et al. reported that compared to the biochars pyrolyzed at high temperatures, biochars derived at low temperatures were more contributive to soil microorganism’s growth because they comprised N and more DOC [29]. Gul et al. described that biochar can indirectly influence the P and N cycling reaction of microbes by altering the soil environment and structure of the microbial community, and can promote the plant rhizobial exchanges [30]. Therefore, adding biochar contributes to soil microbial activity, which may benefit plant growth and development [31]. In general, biochar improves the physicochemical traits of soil and is comprised of nutrients necessary for microbial and plant growth. Hence, biochar application is a potential material for the ecological reclamation of polluted soils with inorganic and organic elements.
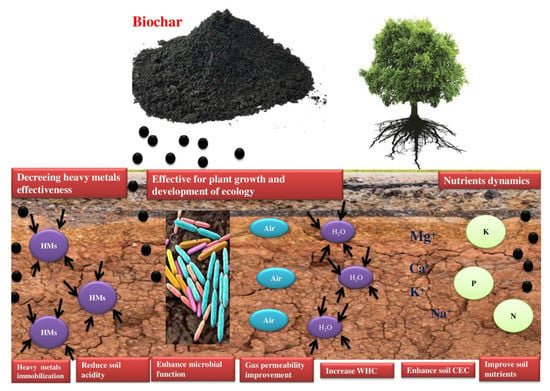
Figure 1. Mechanism of soil amendment by biochar.
2. Biochar Applications for Remediation of Soils Contaminated with Heavy Metals
Heavy metals persist for a long time and are not bio-degradable in polluted soils. The elimination of metals from contaminated soils is time-consuming and expensive. In situ metal stabilization through soil amendments, such as compost and lime, is usually employed to decrease the bio-availability of metals and decrease plant uptake [32]. Biochars can stabilize heavy metals, amend the quality properties of polluted soil, and significantly reduce the uptake of various metals in crops [33]. Thus, biochar application can be a potential solution for the reclamation of soils polluted with heavy metals. Metals stabilization in soils with biochar addition may involve different mechanisms, as explained in Figure 2. Taking lead ions (Pb2+) as an instance, many researchers proposed different mechanisms for the sorption of lead ions through biochar produced from sludge that may include: (i) the exchange of heavy metal with Mg2+, Ca2+, and other cations present in biochar, representing inner-sphere complexation and co-precipitation complexation with mineral oxide and complexed humic matter of biochar; (ii) surface complexation of heavy metals with various functional groups as well as inner-sphere complexation with free-hydroxyl of mineral oxides and other surface precipitation; and (iii) surface precipitation and van der Waals adsorption ensuring the Pb2+ stabilization [34]. In the case of acidic polluted soils, depending on the biochar type and presence of exchangeable cations, such as Ca2+, K+, Mg2+, and Na+ in biochar, these could govern the exchange of cations with heavy metals during the sorption process and may enrich the stabilization process [35]. Ennaji et al. [36] also illustrated that the exchange of heavy metal with K+, Na+, Mg2+, and Ca2+ from sludge-derived biochar was the main process responsible for this exchange in their work, but the contribution of monovalent cations (K+, Na+) was negligible. Thus, it could be stated that under actual field conditions, the biochar-derived sorption process in metal-polluted soils is mainly dependent on soil type and the cations present in both biochar and soils; consequently, metal remediation in polluted soils may differ. Mahmud et al. [37] demonstrated that the mineral constituents, e.g., phosphates and carbonates in the biochar, play a substantial role in stabilizing the metals in soil because these salts can precipitate with metals and lessen their bio-availability. Chen et al. suggested that the primary mechanism for dairy manure-based biochar to retain lead was the precipitation of insoluble lead phosphates [38]. Usually, during biochar preparation, water-soluble Mg, Ca, and P content increase when heated at 200 °C, but these reduced at high temperatures perhaps because of the higher crystallization of P-Mg-Ca. This was evident during the formation of whitlockite when the production temperature was elevated to 400 °C, thereby ensuring the smooth precipitation of lead. Biochar’s alkalinity can also stimulate metal precipitation in the soils [38]. In 2022, Palansooriya investigated the pH variation of the biochar and got a mean value of pH 8.0. With similar biomass materials, the pH value of biochar increases with the preparation temperature due to higher ash contents in the biochar [39]. Thus, many biochars are basic in nature, having a mulching effect that helps decrease the mobility of the heavy metals in polluted soils [40]. Conversely, the removal capacity of the same type of biochar differs with different kinds of heavy metals.
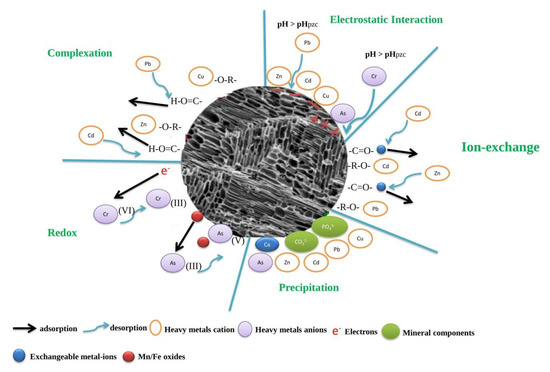
Figure 2. Heavy metals adsorption and immobilization mechanism by biochars in polluted soil.
2.1. Influence of Biochar on the Mobility of Heavy Metals
The application of biochar can decrease the mobility of various heavy metals in polluted soils (Table 1), which minimizes the risk of plant uptake. Various studies have presented that bamboo-derived biochar can remove chromium, nickel, mercury, cadmium, and copper from contaminated soil and water [41]. Biochar obtained from dairy residue prepared at a 300 °C pyrolysis temperature was more effective in sorbing lead than biochar prepared at 400 °C because biochar pyrolyzed at 300 °C had a greater concentration of soluble phosphate [42]. Since biochar properties depend on feedstock type and pyrolysis conditions, a single type of biochar cannot be universally used to reclaim polluted soils containing different heavy metals. Thus, when biochar is to be applied as an amendment for the reclamation of polluted soils, care must be taken about the type of heavy metals, biochar production temperature, residence time, moisture content, and the type of feedstock employed. The influence of biochar on metal bio-availability differs with biochar type and different kinds of heavy metals. Alipour et al. reported that when zinc and cadmium polluted soil was ameliorated by hardwood biochar, the concentration of zinc and cadmium in pore water decreased [8]. Concentrations of extractible zinc and arsenic in soil become higher with the biochar addition rate, whereas the concentration of extractible lead reduced, copper did not modify, and cadmium exhibited an inconsistent trend. They determined that the removal of metals on biochar with primary loadings up to 200 µmol at 7 pH took place in this order: lead > copper > cadmium > zinc > arsenic [43,44]. Singh et al. described that the biochar addition can decrease the discharge of heavy metals due to the redox reaction of heavy metals [44]. For instance, adding chicken manure-derived biochar in chromate-polluted soils increased the decline of mobile chromium hexavalent to less mobile chromium trivalent, thus reducing the leaching of chromium. The reduction in the leaching of chromium trivalent is accredited to adsorption as chromium hydroxide is produced from the release of hydroxide ions (OH−) during the chromium hexavalent reduction mechanism [44] (Figure 2).
Table 1. Effect of different biochars on the mobility of various heavy metals in soil.
| Biochar Type | Application Rate | CEC (cmol/kg) | pH | Pollutant | Effect | Reference |
|---|---|---|---|---|---|---|
| Sugarcane | 1–10% | 69.6 | 9 | Arsenic | Application of sugarcane can decrease concentration of arsenic with the enhance in pH | [30] |
| Beet | --- | --- | 9.5 | Lead, nickel, and cadmium | Beet biochar can efficiently decrease the concentration of various metals in soil, decreasing the amounts of lead, nickel, and cadmium by 87, 26, and 57%, respectively | [33] |
| Hardwood | --- | --- | 9.9 | Zinc and cadmium | Harwood biochar causes enhancement in a soil’s pH, also concentrations of zinc and cadmium in the leachate are decreased by 45 and 300 times | [8] |
| Orange peel | 10% | 29.47 | 10.24 | Cadmium | The 10% application rate of orange peel biochar reduced the concentration of cadmium by 71% | [39] |
| Sludge | 4% | 2.36 | 9.5 | Lead | A 4% biochar addition can reduce lead migration significantly | [16] |
| Lantana and Parthenium | 3% | -- | 8.7 | Chromium, lead, copper, nickel, zinc, iron, and cadmium | Heavy metals’ (Cr, Cd, Cu, Pb, Ni, Zn, Mg, and Fe) bio-accumulation rate and mobility exhibited a significant reduction after biochar application relative to the control | [35] |
| Rice straw | 5% | -- | 9.5 | Zinc, lead, copper, and cadmium | Heavy metals concentrations were significantly lower in rice straw biochar treated soils, 5% rice straw biochar treatment reduced the concentration of zinc, lead, copper, and cadmium by 6, 34, 17, and 11% | [38] |
| Rice straw | 1% | -- | 8.7 | Lead | After biochar addition the concentration of available lead was decreased by 23.6% compared to control | [39] |
| Wheat straw | 5% | 10.4 | 10.6 | Cadmium and lead | The biochar reduced filtrate heavy metals level by 89% to 95% (cadmium) and 93% to 99% (lead) compared with the control | [40] |
| Orchard prunings | 2% | 27.5 | 9.2 | Arsenic, cadmium, copper, lead, and zinc | Biochar increased soil arsenic and metal mobility via changing the soil pH, dissolved organic carbon, and phosphorus | [41] |
| Oak wood | 5% | 24.2 | 10.2 | Lead | Significantly decreased water-soluble, exchangeable, and PBET-extractable lead in soil | [29] |
| Rice husk | 1% | -- | 9.4 | Cadmium, copper, nickel, and zinc | Metal mobility was increased via biochar-introduced dissolved organic carbon | [22] |
| Wood | 1, 2, and 5% | -- | 10.2 | Cadmium | Decrease in cadmium leaching damage by more than 90% | [21] |
| Hardwood | 3% | -- | 8.7 | Zinc and cadmium | Zinc concentration decreased 45- and 300-fold; decrease in cadmium in soil pore water by 10-fold in column leaching tests | [17] |
| Bamboo | 1% | -- | 9.1 | Cadmium | Mutual influence of electro-kinetic, elimination of extractable cadmium by 80% with 2 weeks | [8] |
| Hardwood | 5% | 7.43 | 8.7 | Arsenic, cadmium, copper, lead, and zinc | Biochar surface insulation increased arsenic and copper mobility in soil, little effect on lead and cadmium | [27] |
| Wheat straw | 0.5, 1, and 5% | -- | 10.5 | Cadmium and lead | The biochar addition changed 2.3% to 9.84% of the exchangeable cadmium fraction lead to residual fractions | [13] |
| Stinging nettle | 1–10% | -- | 9.87 | Copper and arsenic | Reduced copper leaching, but affected little on arsenic mobility |
[23] |
| Hardwood | 1% | 24.8 | 9.17 | Cadmium, arsenic, copper, and zinc | Decreased cadmium and zinc while increased arsenic and copper in soil pore water | [4] |
| Eucalyptus wood | 3% | -- | 8.71 | Cadmium | Biochar decreased 0.01 M CaCl2-extractable soil cadmium | [33] |
| Poultry manure | 0.5 and 1% | -- | 10.47 | Cadmium, copper, and lead | NH4NO3-extractable and pore water cadmium and lead reduced in spiked soil; copper, lead, and zinc in plant roots and shoots reduced | [23] |
| Cottonseed hull | 1–10% | -- | 9.67 | Cadmium, copper, nickel, and lead | Greatly reduced the concentrations of all the metals in solution relative to un-amended soil | [20] |
| Poultry litter | 1, 2, and 5% | 11.84 | 8.47 | Copper, cadmium and nickel | Biochar increased Cd and Ni, but reduced Cu sorption by soil. DOM-removed biochar further enhanced all metal sorption | [3] |
| Hardwood | 1–5% | -- | 9.87 | Copper and lead | Significantly decreased soil pore water concentrations of copper and lead | [20] |
| Hardwood | 1% | 17.48 | 10.01 | Nickel and zinc | Biochar decreased metal leaching by 80% and enhanced the residual portion in soil | [14] |
2.2. Influence of Biochar on Heavy Metals Bio-Availability
The bio-availability of various metals indicates the toxicity in soils and the potential hazard of contaminating the human food-web. The bio-availability of contaminants regulates their degradation and eco-toxicology in polluted soils. Bio-availability is defined as a pollutant fraction representing the availability of a chemical agent to a living organism for eco-toxicology, assimilation, and degradation expression [33]. Many studies showed that applying biochar is more efficient in immobilizing heavy metals, thereby decreasing their phytotoxicity and bio-availability (Table 2). Liu et al. assessed the ability of biochar addition to amend the heavy metals toxicity in pit-tailings [45]. They used biochar prepared from orchard prune residues at 0%, 1%, 5%, and 10% rates. WHC, CEC, and pH level were increased with increasing biochar application rates, and the bio-availability of zinc, lead, and cadmium of mine-tailings was reduced, while cadmium showed the maximum reduction. According to Montagnoli et al. [18], applied biochar produced from cotton stalks improved the cadmium-polluted soil. The findings suggested that biochar obtained from cotton stalks can decrease the bio-availability of soil cadmium by co-precipitation or an adsorption mechanism. According to another study, the effects of sewage sludge-derived biochar on metals bio-availability and solubility in Mediterranean farming soil were compared with untreated sewage sludge (not charred). The biochar applications decreased the plant accessibility of lead, cadmium, zinc, and nickel when equated to sewage application [46]. Table 2 summarizes the outcome of various biochars on the uptake volume of pollutants and the bio-availability of contaminants. Biochar produced from green waste and chicken manure significantly reduced lead, copper, and cadmium uptake by Brassica juncea. It was also found that the decline in plant metal concentration was increased with increasing biochar rates, except for copper concentration. Biochar produced from rice proved more effective to immobilize lead and copper than cadmium [47]. Hence, when the sole objective of biochar addition is to immobilize various metals, special attention should be paid to selecting suitable feedstock and the production temperature of biochar. Gamboa et al. conducted a pot experiment and used activated biochar of wood in the soil spiked with metals to examine the biochar’s effect on the accessibility of zinc, lead, copper, and cadmium to corn [48]. Biochar addition reduced the concentration of copper, cadmium, and arsenic in corn shoots, but the effect of biochar addition was inconsistent on zinc and lead concentrations in corn shoots. Soil pH is closely associated with the bio-availability of metals in the soil. The addition of biochar can improve the CEC and pH of soil, and consequently increase the immobilization of various metals in the soil [49]. Siles et al. [50] conducted a study using biochar obtained from cow manure and mussel shell to decrease the lead toxicity in prominently lead-polluted soil in South Korea. Lead bio-availability in soil was reduced by 76% with biochar application. An increase in adsorption capacity and a rise in soil pH were considered the result of the reclamation effect of biochar. For instance, lead bio-availability in soil was reduced up to 93% with shell biochar, a mulching material. At present, many studies revealed that various kinds of biochars can decrease heavy metals’ bio-availability and their mobility. However, most of this research is carried out under controlled environments (under greenhouse and laboratory experiments). Therefore, to fully utilize the biochar potential as a reclamation agent, large-scale field studies should be conducted.
Table 2. Effect of biochar addition on bio-availability of heavy metals in soils.
| Biochar | Preparation Temperature (°C) | Heavy Metals | Outcome | Reference |
|---|---|---|---|---|
| Chicken waste | 550 | Chromium | Increased soil Cr(IV) reduction to Cr(III) | [18] |
| Eucalyptus | 500 | Zinc, cadmium, copper, and arsenic | Reduction in zinc, cadmium, copper, and arsenic in corn shoots | [20] |
| Sewage sludge | 550 | Zinc, lead, nickel, copper, and cadmium | Substantial decrease in plant availability of these metals | [38] |
| Hardwood | 400 | Arsenic | Noteworthy reduction of arsenic in foliage of the Silver-grass | [29] |
| Chicken waste | 500 | Lead, copper, and cadmium | Notable decrease of lead, copper, and cadmium accumulation by Brassica juncea | [14] |
| Rice straw | 450 | Lead, copper, and cadmium | Substantial decrease in concentration of lead, copper, and cadmium in polluted soil | [3] |
| Orchard residue | 600 | Lead, copper, cadmium, and zinc | Notable decrease of bio-available lead, copper, cadmium, and zinc, with cadmium showing utmost reduction | [35] |
| Maize straw | 550 | Cadmium | Decrease of bio-availability of cadmium in soil through co-precipitation or adsorption process | [18] |
| Wheat straw | 450 | Cadmium and lead | Bio-available cadmium and lead were reduced by 4.48% to 10.69% (Cd) and 11.74% to 16.42% (Pb) in surface soil (0 to 4 cm) | [34] |
| Hardwood | 400 | Cadmium, lead, and arsenic | Reduced cadmium and zinc concentrations, but not arsenic in soil leachate | [48] |
| Poultry litter | 350 | Copper, cadmium, and nickel | Biochar enhanced cadmium and nickel, but decreased copper sorption via soil. Dissolved organic matter-removed biochar further increased all metal sorption | [19] |
| Rice straw | 500 | Cadmium, lead, and zinc | Biochar decreased soil bio-available and vegetable metals and enhanced plant biomass yield | [36] |
| Oak wood charcoal | 450 | Cadmium and copper | Charcoal reduced soil-available, leachable, and bio-accessible cadmium and copper | [39] |
| Rice straw | 350 | Cadmium | Soil pH increased, exchangeable cadmium reduced, but Fe-oxide and OM-bound cadmium enhanced | [17] |
| Rice husk | 500 | Mercury | Rice husk feedstock can expressively decrease the transport of mercury in soil | [50] |
| Poultry manure | 400 | Copper | Decrease the concentration of Cu in soil pore water and soil, diminish the transferable contents of Cu in the plants, and enhances the residual state in plants contents as well as organic substance binding | [26] |
| Fruit bunches | 550 | Lead, copper, and cadmium | When the application rate was 20%, the content of Cd in brassica aerial parts reduced by around 90% and Pb content reduced by 95% as well as copper content reduced by 63% | [18] |
| Oak branches | 500 | Lead | Pb bio-availability in soil reduced by 15 and 76% | [50] |
| Orchard residue | 500 | Arsenic | Arsenic components in roots of tomato reduced by around 70% | [14] |
| Wheat straw | 450 | Cadmium and lead | Concentration of bio-availability of cadmium and lead was decreased 13.84% to 16.15% and 4.02% to 13.40% in 4 to 8 cm soil | [32] |
| Miscanthus | 700 | Copper, lead, zinc, and cadmium | pH changes upon biochar amendment, the results exhibited that biochar decreased extractability of copper, lead, and zinc, but not of Cd | [50] |
| Rice straw | 500 | Cadmium, zinc, lead, and arsenic | Biochar reduced cadmium, zinc, and lead, but increased arsenic in soil pore water and rice | [41] |
| Orchard prunings | 350 | Arsenic, cadmium, copper, lead, and zinc | Reduced free metals yet elevated arsenic and dissolved organic carbon-associated metals in soil pore water | [22] |
| Sewage sludge | 450 | Arsenic, cadmium, cobalt, chromium, copper, nickel, lead, and zinc | Decreased soil EDTA-extractable and bio-accumulated arsenic, chromium, cobalt, nickel, and lead, but increased the portions of others | [39] |
| Soybean straw | 300 | Copper, lead, and antimony | Biochar immobilized lead and copper, but mobilized antimony | [25] |
| Rice straw | 350 | Cadmium | Lettuce cadmium content decreased in lightly contaminated but not in heavily contaminated soil | [42] |
This entry is adapted from the peer-reviewed paper 10.3390/separations10030197
This entry is offline, you can click here to edit this entry!
