Adipose tissue is contemplated as a dynamic organ that plays key roles in the human body. Adipogenesis is the process by which adipocytes develop from adipose-derived stem cells to form the adipose tissue. Adipose-derived stem cells’ differentiation serves well beyond the simple goal of producing new adipocytes. Indeed, with the current immense biotechnological advances, the most critical role of adipose-derived stem cells remains their tremendous potential in the field of regenerative medicine. This entry focuses on examining the physiological importance of adipogenesis, the current approaches that are employed to model this tightly controlled phenomenon, and the crucial role of adipogenesis in elucidating the pathophysiology and potential treatment modalities of human diseases. The future of adipogenesis is centered around its crucial role in regenerative and personalized medicine.
- Adipogenesis
- Adipose Tissue
- Cell Modelling
- 2D Culture
- 3D Culture
- Obesity
1. Introduction
Adipose tissue is often regarded as a dynamic organ with primordial functions that underline its physiological value. Its versatile contribution to the human body functions include lipid storage, energy homeostasis, and a major share in insulin and other hormonal signaling. Adipose tissue can be classically classified into two different entities: white and brown adipose tissue [1]. Other separate entities also exist, including beige/brite adipose tissue, perivascular adipose tissue, and bone marrow adipose tissue [2]. White adipose tissue represents the largest share of fat that is usually present in the adult human body and is mainly responsible for the aforementioned functions [1]. As a matter of fact, adipokine and cytokine secretion underlines the role of white fat as an endocrine tissue in itself [3]. Brown adipose tissue, on the other hand, is notably abundant in newborns and hibernating mammals. Although adipose tissue encompasses a multitude of cells (macrophages, blood cells, fibroblasts, endothelial cells, and stem cells), mature adipocytes remain the most abundant cell type. It is now well-appreciated that brown and white adipocytes originate from distinct precursor cells. The process by which adipocytes develop from adipose-derived stem cells to form the adipose tissue is called adipogenesis. Adipose-derived stem cells’ differentiation serves well beyond the simple goal of producing new adipocytes. In fact, with the current immense biotechnological advances, the most critical role of adipose-derived stem cells remains their tremendous potential in the field of regenerative and personalized medicine.
2. Adipogenesis and Human Disease
In terms of human diseases, it is worth noting that adipogenesis is not exclusively limited to portraying obesity. In fact, adipogenesis has been employed as a model for a multitude of diseases [4]. When it comes to obesity, it has become a worldwide critical public health burden recently. It has been estimated that, by 2030, 38% of the world’s adults population will be overweight, and 20% of them will be obese [5]. The excess fat mass can be the result of both hypertrophy (increase in cell size) and hyperplasia (increase in cell number) of adipocytes in white adipose tissue [6]. The interplay between the two adipose tissue types plays a key role in regulating obesity. The inflammatory processes in white adipose tissue is a precursor to oxidative stress and the consequent insulin resistance that alters the systemic homeostasis, thus leading to the metabolic syndrome. This is in opposition to brown adipose tissue that is heavily implicated in thermogenesis and energy expenditure. The latter is controlled by the mitochondrial uncoupling protein 1 (UCP-1) [7]. Interestingly, upper-body adiposity is clearly distinct from lower-body adiposity, with the former being a risk factor for obesity and the latter being protective against obesity. Preadipocyte cellular models have been established to further investigate this difference [8]. When it comes to diseases other than obesity, it has been reported that adipose tissue models can be used to study diseases such as cancer and type 2 diabetes mellitus. The impaired insulin signaling forms a tight link between obesity and type 2 diabetes mellitus, making adipocytes a suitable model for the investigation of the disease’s pathophysiology [9]. To note, the isoform-2 of peroxisome proliferator-activated receptor gamma (PPAR-γ2) is one of the major transcription factors that are present in adipose tissue and plays a primordial role in the differentiation process. It was shown to be involved in a variety of metabolic disturbances, such as insulin resistance, dyslipidemia, type 2 diabetes mellitus, and subsequently obesity [10]. Adipogenesis has been also employed to model cancers, such as breast cancer [11][12], prostate cancer [13][14][15], and multiple myeloma [16].
2.1. Stem Cells and Adipogenesis
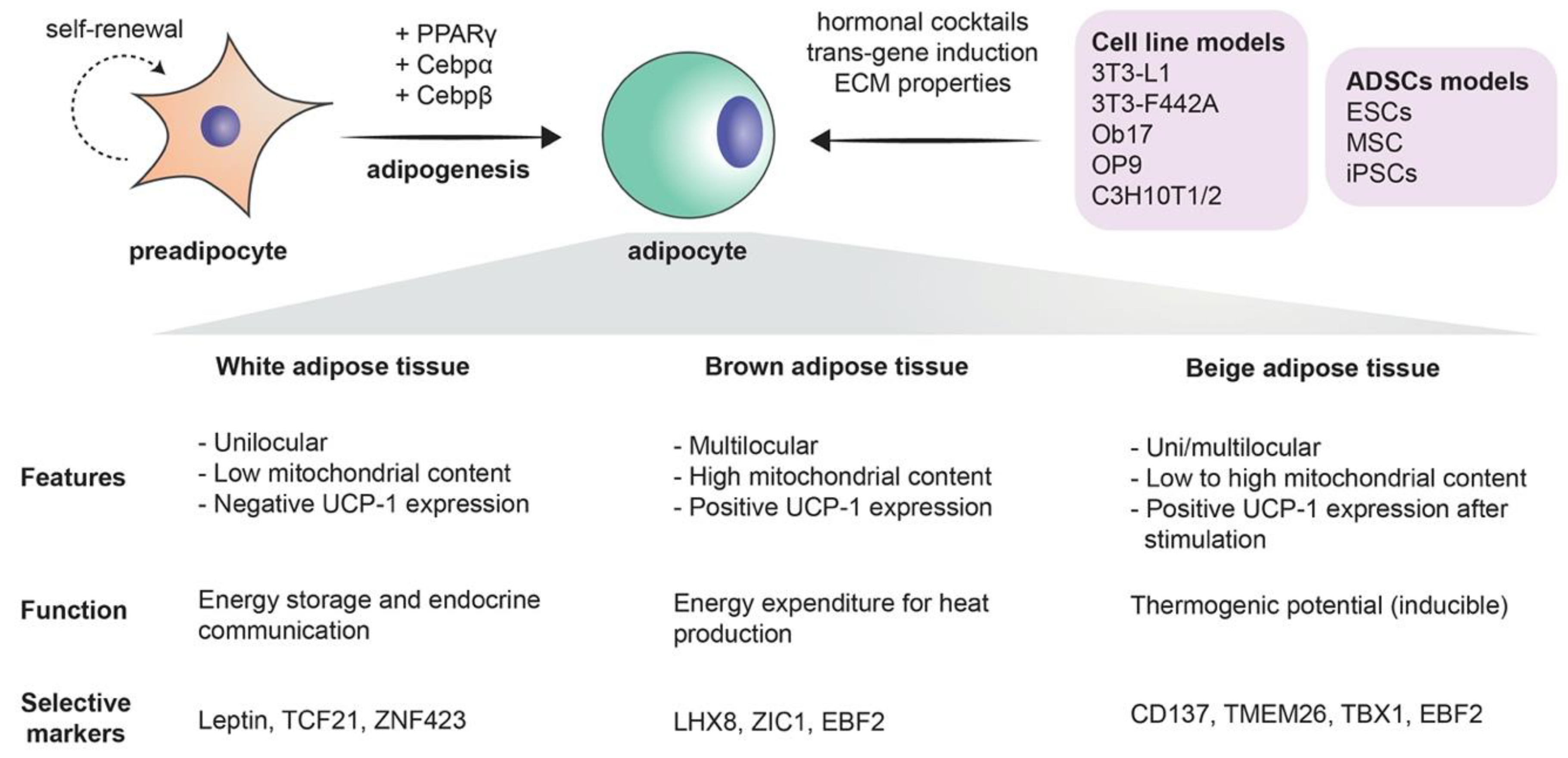
Mesenchymal stem cells are the precursors of adipocytes. These cells differentiate into lipoblasts, then into preadipocytes, and ultimately into the mature adipocytes. Briefly, when adipogenesis takes place, the fibroblast-like preadipocytes differentiate into insulin-responsive adipocytes [17]. The differentiation process is a complex process in which many transcription factors are involved, such as peroxisome proliferator-activated receptor γ (PPARγ), CCAAT/enhancer-binding proteins (C/EBPs), Krüppel-like factor (KLF), and proteins signal transducers and activators of transcription (STATs) [1] (Figure 1). The existence of adipose stem cells is in no way a novel finding: as a matter of fact, it is supported by a large share of evidence. The sole existence of an “adipostat” (fat homeostasis) that is maintained by the pool of adipose stem cells is part of this evidence. Human pathologies like progressive osseous heteroplasia, in which ectopic bone arises from the subcutaneous adipose layer of the skin, also prove the possible “tripotency” of adipose stem cells that can give rise to chondrogenic, osteogenic, and adipogenic cell lines. Finally, the treatment of liposarcomas with ligands that target the previously mentioned PPARγ implies that liposarcomas originate from stem cells, as in the process, liposarcoma cells undergo adipogenesis [18]. Furthermore, the use of adipose-derived stem cells extends beyond the realm of adipocytes alone, as it has been demonstrated that these stem cells can be differentiated into endothelial cells. This further supports the solid crosstalk between adipogenesis and angiogenesis [19]. More importantly, with the current immense biotechnological advances, the most critical role of adipose-derived stem cells remains their tremendous potential in the field of regenerative medicine [20].
2.2. Immune Cell Adipocyte Crosstalk
There is considerable clinical evidence that obesity, specifically in combination with type 2 diabetes mellitus (T2DM), causes increased prevalence of a plethora of medical conditions that are immune-mediated. For example, common infections reoccur with higher frequency, exacerbated with increased severity that potentially leads to other complications [24][25]. Higher rates of vaccine failure have also been reported in individuals with obesity [26], perhaps due to compromised adaptive immunity. In all, the association of obesity with a compromised immune system, and a state of chronic low-grade inflammation in adipose tissue [27] is an indication of some degree of cross talk between adipocytes and cells of the immune system. Along with other cell types, such as endothelial and fibroblasts cells, lean adipose tissue contains macrophages for immunologic surveillance purposes. Obese adipose tissue however, can consists of up to 40% of pro-inflammatory macrophages, along with T-cells and B-cells [28][29]. Overall, levels of pro-inflammatory cytokines, TNFα, IL-6, and MCP-1 increased when 3T3-L1 cells were cocultured with murine splenocytes, using a Transwell culture system [29] recapitulating possible cell–cell interaction scenarios in adipose tissues. Interestingly, IL-6 and MCP-1 measured higher when adipocytes and immune cells were in direct contact when activated via lipopolysaccharide (LPS), TNFα measured higher only when exposed to immune-cell-conditioned media. Splenocytes contain a mixed population of cells, while this potentially recapitulates the in vivo situation, the interaction of macrophages and adipocytes alone cannot be elucidated. Using LPS-activated macrophages derived from monocytes purified from human donors, Sarvari et al. [28] showed that IL-6 is macrophage-dependent, as a result of phagocytosis of adipocytes. They also observed lipid droplet accumulation within macrophages after adipocyte–macrophage co-culture [28], which could be a result of the engulfment or uptake of soluble lipids during the phagocytic activity. Garcia-Sabate et al. [30] recently showed that macrophages in 3D mono-culture are able to uptake exogenous low-density lipoprotein and with lipid droplet accumulation dependent on the cell phenotype [30]. Alongside pro-inflammatory cytokines, they also detailed adipokine and growth factor release as a result of lipid accumulation, and, interestingly, levels are dependent not only on macrophage phenotype but on substrate density, as well. Adipocytes are also able to act as antigen-presenting cells, through major histocompatibility complex II, to stimulate IFNγ expressing T-cells [31], which are implicated in autoimmune diseases. This finding is reserved only for large adipocytes of a certain size. The modulation of T-cells correlates with insulin resistance and the involvement of T-cells during obesity is nicely reviewed by Nyambuma et al. 2019 [32]. Despite adipose tissue being known to attract immune cells to proximity, little is known regarding their interactions [28], warranting further research in this field.
2.3. Modeling Adipogenesis via 2D and 3D In Vitro Models and In Vivo Animal Models
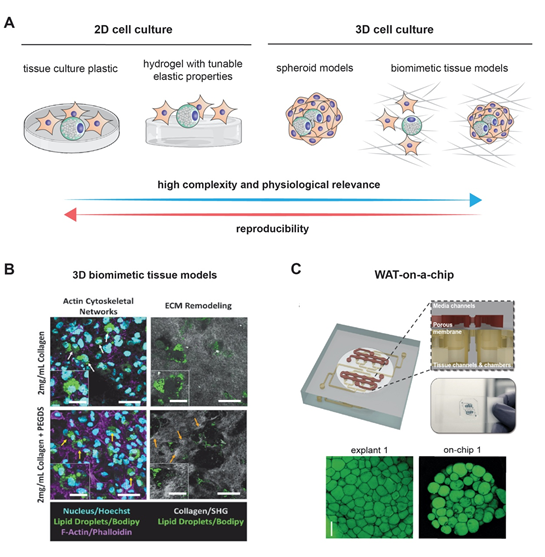
Modeling the growth of adipocytes in vitro has been extensively studied in the recent years [4] (Figure 1). Notably, the 3T3-L1 cell line, which can differentiate from fibroblasts to adipocytes, remains one of the most frequently used cell lines with standardized and readily available protocols [33]. Two-dimensional (2D) models, however, often fail to precisely replicate the true complexity of adipogenesis. Animal models that are characterized by an extensive lipid deposition in skeletal muscles that is often seen in several human pathologies like myopathies may be considered as acceptable models for studying the mechanisms behind adipogenesis. Wagyu cattle represent a notable example [34]. As much as they are useful, animal models have many drawbacks, including their high cost, their time-consuming isolation procedures, and their failure to recapitulate human pathophysiology due to species differences [35]. Fortunately, it is worth noting that the fidelity in modeling human adipocytes has further improved with the use of human preadipocytes and the previously mentioned adipose-derived stem cells. Add to that the rich interaction of adipocytes with their environment is no longer a secret after the use of 3D models and co-cultures [4][36]. Naturally occurring and biocompatible silk protein scaffolds offered a unique advantage in bioactive adipose tissue engineering [36]. One of the major advancements in culture techniques is the employment of scaffold-free methods in which 3D adipose spheroids are generated from immortal mouse or human pre-adipocyte. Three-dimensional spheroids have been shown to have a more abundant expression and secretion of adiponectin as compared to 2D culture. Their ability to secrete pro-inflammatory cytokines equips them with a superior ability of resisting culture or toxin associated stress. Finally, 3D spheroids that are generated from brown adipose tissue have a higher retention of brown adipose tissue markers than the classical 2D cultures cells from the same origin [35]. It is worth noting here that the superior characteristics of 3D cultures were exploited to model breast cancer, as previously mentioned [12]. An figure highlighting the potential application of both culturing methods is displayed in Figure 2.
Figure 2. Modeling adipogenesis: (A) cell culture models used to study adipogenesis in vitro. Although 2D cell culture models are simple and reproducible, they lack complexity and physiological relevance as exhibited in 3D cell culture models. Example of complex 3D cell culture models (B) using 3D collagen matrices as a biomimetic tissue model, and (C) a white adipose tissue (WAT)-on-a-chip. Images are adapted with permission from [37] and [38], for Figure 2B and 2C, respectively.
3. Clinical Implications of Adipogenesis Models
The potential contribution of adipogenesis in the elucidation of the pathophysiology of multiple diseases via constituting a biologically representative model is not its only outstanding attribute. As a matter of fact, adipogenesis may be implicated in the treatment of various human disease. For instance, adipogenesis models could be utilized for lipidomics studies in various clinically relevant areas, including diabetes [39][40], cardiovascular disease [41][42], prostate cancer [43][44], and psychiatric diseases such as schizophrenia [43].
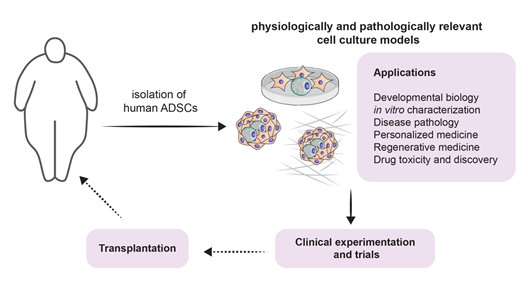
It is true that the involvement of adipogenesis in the novel and scientifically appealing field of regenerative medicine forms a broad conglomeration of potential treatment strategies [18] (Figure 3). However, one must acknowledge that the therapeutic potential of adipogenesis extends beyond this field. Beige fat was shown to be heavily implicated in energy expenditure. This has been exploited for therapeutic purposes, especially when it comes to obesity: White-to-beige adipocyte conversion is probably one of the main processes that orchestrate this therapy [44]. It is also known that the accumulation of fat in visceral adipose tissue is one of the risk factors for increasing insulin resistance and thus accelerating the progression of type 2 diabetes mellitus. Dysfunctional adipogenesis of omental adipose tissue and high levels of 4-hydroxynonenal (4-HNE) are key players in this phenomenon. The in vitro combination of metformin and insulin was shown to decrease the adipogenesis impairment of preadipocytes that are derived from type 2 diabetes mellitus patients [45].
Figure 3. Modeling adipogenesis for experimental approaches and clinical applications: A schematic illustrating potential experimental approaches from isolation of ADSCs throughout culturing, applications (listed), including clinical experiments and trial, and finally towards potential transplantation.
This entry is adapted from the peer-reviewed paper 10.3390/cells9102326
References
- Sarjeant, K.; Stephens, J.M. Adipogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008417.
- Murphy, C.S.; Liaw, L.; Reagan, M.R. In vitro tissue-engineered adipose constructs for modeling disease. BMC Biomed. Eng. 2019, 1, 27.
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97.
- Ruiz-Ojeda, F.J.; Rupérez, A.I.; Gomez-Llorente, C.; Gil, A.; Aguilera, C.M. Cell Models and Their Application for Studying Adipogenic Differentiation in Relation to Obesity: A Review. Int. J. Mol. Sci. 2016, 17, 1040.
- Kelly, T.; Yang, W.; Chen, C.S.; Reynolds, K.; He, J. Global burden of obesity in 2005 and projections to 2030. Int. J. Obes. 2008, 32, 1431–1437.
- Wang, Q.A.; Scherer, P.E.; Gupta, R.K. Improved methodologies for the study of adipose biology: Insights gained and opportunities ahead. J. Lipid Res. 2014, 55, 605–624.
- Boyer, W.R.; Johnson, T.M.; Fitzhugh, E.C.; Richardson, M.R.; Churilla, J.R. The associations between increasing degrees of homeostatic model assessment for insulin resistance and muscular strengthening activities among euglycaemic US adults. Diabetes Vasc. Dis. Res. 2015, 12, 420–427.
- Todorčević, M.; Hilton, C.; McNeil, C.; Christodoulides, C.; Hodson, L.; Karpe, F.; Pinnick, K.E. A cellular model for the investigation of depot specific human adipocyte biology. Adipocyte. 2017, 6, 40–55.
- Abdullah, A.; Peeters, A.; de Courten, M.; Stoelwinder, J. The magnitude of association between overweight and obesity and the risk of diabetes: A meta-analysis of prospective cohort studies. Diabetes Res. Clin. Pract. 2010, 89, 309–319.
- Saraf, N.; Sharma, P.K.; Mondal, S.C.; Garg, V.K.; Singh, A.K. Role of PPARg2 transcription factor in thiazolidinedione-induced insulin sensitization. J. Pharm. Pharmacol. 2012, 64, 161–171.
- Delort, L.; Lequeux, C.; Dubois, V.; Dubouloz, A.; Billard, H.; Mojallal, A.; Damour, O.; Vasson, M.P.; Caldefie-Chézet, F. Reciprocal interactions between breast tumor and its adipose microenvironment based on a 3D adipose equivalent model. PLoS ONE. 2013, 8, e66284.
- Ding, Y.; Liu, W.; Yu, W.; Lu, S.; Liu, M.; Kaplan, D.L.; Wang, X. Three-dimensional tissue culture model of human breast cancer for the evaluation of multidrug resistance. J. Tissue Eng. Regen. Med. 2018, 12, 1959–1971.
- Herroon, M.K.; Diedrich, J.D.; Podgorski, I. New 3D-Culture Approaches to Study Interactions of Bone Marrow Adipocytes with Metastatic Prostate Cancer Cells. Front. Endocrinol. 2016, 7, 84.
- Mosaad, E.; Chambers, K.; Futrega, K.; Clements, J.; Doran, M.R. Using high throughput microtissue culture to study the difference in prostate cancer cell behavior and drug response in 2D and 3D co-cultures. BMC Cancer 2018, 18, 592.
- Wang, X.; Reagan, M.R.; Kaplan, D.L. Synthetic adipose tissue models for studying mammary gland development and breast tissue engineering. J. Mammary Gland. Biol. Neoplasia 2010, 15, 365–376.
- Reagan, M.R.; Mishima, Y.; Glavey, S.V.; Zhang, Y.; Manier, S.; Lu, Z.N.; Memarzadeh, M.; Zhang, Y.; Sacco, A.; Aljawai, Y.; et al. Investigating osteogenic differentiation in multiple myeloma using a novel 3D bone marrow niche model. Blood 2014, 124, 3250–3259.
- Gregoire, F.M.; Smas, C.M.; Sul, H.S. Understanding adipocyte differentiation. Physiol. Rev. 1998, 78, 783–809.
- Gimble, J.M.; Katz, A.J.; Bunnell, B.A. Adipose-derived stem cells for regenerative medicine. Circ. Res. 2007, 100, 1249–1260.
- Volz, A.C.; Huber, B.; Kluger, P.J. Adipose-derived stem cell differentiation as a basic tool for vascularized adipose tissue engineering. Differentiation. 2016, 92, 52–64.
- Frese, L.; Dijkman, P.E.; Hoerstrup, S.P. Adipose Tissue-Derived Stem Cells in Regenerative Medicine. Transfus. Med. Hemother. 2016, 43, 268–274.
- Nagy, L.; Rauch, B.; Balla, N.; Ujlaki, G.; Kis, G.; Abdul-Rahman, O.; Kristof, E.; Sipos, A.; Antal, M.; Toth, A.; et al. Olaparib induces browning of in vitro cultures of human primary white adipocytes. Biochem. Pharmacol. 2019, 167, 76–85.
- Klusoczki, A.; Vereb, Z.; Vamos, A.; Fischer-Posovszky, P.; Wabitsch, M.; Bacso, Z.; Fesus, L.; Kristof, E. Differentiating SGBS adipocytes respond to PPARgamma stimulation, irisin and BMP7 by functional browning and beige characteristics. Sci. Rep. 2019, 9, 5823.
- Toth, B.B.; Arianti, R.; Shaw, A.; Vamos, A.; Vereb, Z.; Poliska, S.; Gyory, F.; Bacso, Z.; Fesus, L.; Kristof, E. FTO Intronic SNP Strongly Influences Human Neck Adipocyte Browning Determined by Tissue and PPARgamma Specific Regulation: A Transcriptome Analysis. Cells 2020, 9, 987.
- Ackerman, J.E.; Geary, M.B.; Orner, C.A.; Bawany, F.; Loiselle, A.E. Obesity/Type II diabetes alters macrophage polarization resulting in a fibrotic tendon healing response. PLoS ONE 2017, 12, e0181127.
- Richard, C.; Wadowski, M.; Goruk, S.; Cameron, L.; Sharma, A.M.; Field, C.J. Individuals with obesity and type 2 diabetes have additional immune dysfunction compared with obese individuals who are metabolically healthy. BMJ Open Diabetes Res. Care. 2017, 5, e000379.
- Andersen, C.J.; Murphy, K.E.; Fernandez, M.L. Impact of Obesity and Metabolic Syndrome on Immunity. Adv. Nutr. 2016, 7, 66–75.
- Zhou, H.; Liu, F. Regulation, Communication, and Functional Roles of Adipose Tissue-Resident CD4(+) T Cells in the Control of Metabolic Homeostasis. Front. Immunol. 2018, 9, 1961.
- Sarvari, A.K.; Doan-Xuan, Q.M.; Bacso, Z.; Csomos, I.; Balajthy, Z.; Fesus, L. Interaction of differentiated human adipocytes with macrophages leads to trogocytosis and selective IL-6 secretion. Cell Death Dis. 2015, 6, e1613.
- Nitta, C.F.; Orlando, R.A. Crosstalk between immune cells and adipocytes requires both paracrine factors and cell contact to modify cytokine secretion. PLoS ONE 2013, 8, e77306.
- Garcia-Sabate, A.; Mohamed, W.K.E.; Sapudom, J.; Alatoom, A.; Al Safadi, L.; Teo, J.C.M. Biomimetic 3D Models for Investigating the Role of Monocytes and Macrophages in Atherosclerosis. Bioengineering 2020, 7, 113.
- Xiao, L.; Yang, X.; Lin, Y.; Li, S.; Jiang, J.; Qian, S.; Tang, Q.; He, R.; Li, X. Large adipocytes function as antigen-presenting cells to activate CD4(+) T cells via upregulating MHCII in obesity. Int. J. Obes. 2016, 40, 112–120.
- Nyambuya, T.M.; Dludla, P.V.; Mxinwa, V.; Nkambule, B.B. Obesity-induced inflammation and insulin resistance: A mini-review on T-cells. Metabol. Open 2019, 3, 100015.
- Zebisch, K.; Voigt, V.; Wabitsch, M.; Brandsch, M. Protocol for effective differentiation of 3T3-L1 cells to adipocytes. Anal. Biochem. 2012, 425, 88–90.
- Campos, C.F.; Duarte, M.S.; Guimarães, S.E.; Verardo, L.L.; Wei, S.; Du, M.; Jiang, Z.; Bergen, W.G.; Hausman, G.J.; Fernyhough-Culver, M.; et al. Review: Animal model and the current understanding of molecule dynamics of adipogenesis. Animal 2016, 10, 927–932.
- Klingelhutz, A.J.; Gourronc, F.A.; Chaly, A.; Wadkins, D.A.; Burand, A.J.; Markan, K.R.; Idiga, S.O.; Wu, M.; Potthoff, M.J.; Ankrum, J.A. Scaffold-free generation of uniform adipose spheroids for metabolism research and drug discovery. Sci. Rep. 2018, 8, 523.
- Choi, J.H.; Bellas, E.; Vunjak-Novakovic, G.; Kaplan, D.L. Adipogenic differentiation of human adipose-derived stem cells on 3D silk scaffolds. Methods Mol. Biol. 2011, 702, 319–330.
- Turner, P.A.; Gurumurthy, B.; Bailey, J.L.; Elks, C.M.; Janorkar, A.V. Adipogenic Differentiation of Human Adipose-Derived Stem Cells Grown as Spheroids. Process Biochem. 2017, 59, 312–320.
- Turner, P.A.; Tang, Y.; Weiss, S.J.; Janorkar, A.V. Three-dimensional spheroid cell model of in vitro adipocyte inflammation. Tissue Eng. Part A 2015, 21, 1837–1847.
- Lappas, M.; Mundra, P.A.; Wong, G.; Huynh, K.; Jinks, D.; Georgiou, H.M.; Permezel, M.; Meikle, P.J. The prediction of type 2 diabetes in women with previous gestational diabetes mellitus using lipidomics. Diabetologia 2015, 58, 1436–1442.
- Meikle, P.J.; Wong, G.; Barlow, C.K.; Kingwell, B.A. Lipidomics: Potential role in risk prediction and therapeutic monitoring for diabetes and cardiovascular disease. Pharmacol. Ther. 2014, 143, 12–23.
- De Leon, H.; Boue, S.; Szostak, J.; Peitsch, M.C.; Hoeng, J. Systems Biology Research into Cardiovascular Disease: Contributions of Lipidomics-based Approaches to Biomarker Discovery. Curr. Drug. Discov. Technol. 2015, 12, 129–154.
- Hinterwirth, H.; Stegemann, C.; Mayr, M. Lipidomics: Quest for molecular lipid biomarkers in cardiovascular disease. Circ. Cardiovasc. Genet. 2014, 7, 941–954.
- McEvoy, J.; Baillie, R.A.; Zhu, H.; Buckley, P.; Keshavan, M.S.; Nasrallah, H.A.; Dougherty, G.G.; Yao, J.K.; Kaddurah-Daouk, R. Lipidomics reveals early metabolic changes in subjects with schizophrenia: Effects of atypical antipsychotics. PLoS ONE 2013, 8, e68717.
- McQueen, A.E.; Koliwad, S.K.; Wang, J.C. Fighting obesity by targeting factors regulating beige adipocytes. Curr. Opin. Clin. Nutr. Metab. Care. 2018, 21, 437–443.
- Jaganjac, M.; Almuraikhy, S.; Al-Khelaifi, F.; Al-Jaber, M.; Bashah, M.; Mazloum, N.A.; Zarkovice, K.; Zarkovicf, N.; Waegg, G.; Kafienah, W.; et al. Combined metformin and insulin treatment reverses metabolically impaired omental adipogenesis and accumulation of 4-hydroxynonenal in obese diabetic patients. Redox. Biol. 2017, 12, 483–490.