2. Radiotherapy-Based Treatment
2.1. Conventional Radiotherapy Alone
Radiation therapy has been used to treat unresectable breast cancer or elderly patients. Conventional fractionation or hypofractionation with conventional doses was used, so such treatments were not definitive ones. Previous data indicated that with conventional radiation doses, 3-year local control rates would be expected to be 45–57% [
8,
9,
10]; these rates are insufficient as a definitive treatment. In a relatively large retrospective analysis of 192 patients with locally advanced breast cancer, patients were treated with 45–50 Gy to the breast, and about 80% of the patients received a local boost. Furthermore, 28% of the patients received multi-agent chemotherapy. As a result, however, the 5-year local control rate was 73%, and 5-year survival was 41% [
11]. In another study of early breast cancer patients treated by primary radiotherapy, 27 tumors were found to have histologic features of moderate to marked intraductal carcinoma in the tumor and adjacent tissue and a high nuclear grade; the 5-year local control rate was 84% for 15 patients receiving ≥60 Gy, whereas it was 48% for those who received <60 Gy [
12]. Hypofractionated treatment was used for more palliative cases [
13], so data from such treatment are not fully evaluable. Combination with adjuvant hormone therapy would improve the survival time of hormone-receptor-positive patients [
13].
2.2. Concurrent Chemoradiotherapy
Since conventional radiotherapy alone is insufficient to achieve a high local control rate, concurrent chemoradiotherapy has been investigated for early breast cancer by a few groups [
14,
15,
16]. In a case series of 5 patients, 4 achieved local control for more than 2.5 years, but 1 developed local recurrence, which was treated by reirradiation [
14]. The Japanese Clinical Oncology Group investigated preoperative chemoradiation for 108 Stage I–IIIA patients. All patients underwent mastectomy or lumpectomy thereafter, and a pathological complete response was achieved in only 36% of the patients [
15]. Therefore, the conclusion of the study was that the treatment was not sufficient to use as a definitive treatment. In locally advanced breast cancer patients, concurrent chemoradiation was investigated by many groups in a preoperative neoadjuvant setting [
16,
17,
18]; surgery was a prerequisite of treatment, and cure was not a goal of chemoradiation. Histological evaluation of resected specimens showed pathological complete response rates of 9–61% (median, 29%) [
16]. Concurrent chemoradiotherapy in combination with neoadjuvant/adjuvant chemotherapy and hormonal therapy when indicated should be a method of definitive treatment, but intensification of radiation therapy may be necessary to achieve a high enough local control rate.
2.3. Radiotherapy with Hydrogen Peroxide Sensitization
As a definitive treatment for early breast cancer, Ogawa et al. [
19] developed a new treatment modality named KORTUC (Kochi Oxydol-Radiation Therapy for Unresectable Carcinomas). In this treatment, hydrogen peroxide was injected into the breast tumor just before radiation therapy [
20,
21]. Hydrogen peroxide produces oxygen in the tumor and, hence, sensitizes hypoxic tumor cells to radiotherapy [
22]. In addition, hydrogen peroxide inactivates anti-oxidative enzymes, such as peroxidases and catalases that are scavengers of radicals produced by radiation and reduce the therapeutic efficacy of RT [
19]. Usually, hydrogen peroxide dissolved in sodium hyaluronate was injected twice a week, while radiation was delivered five times a week. Radiation doses used by Ogawa’s group were 44 Gy in 16 fractions (2.75 Gy per day) to the whole breast, followed by an electron boost with 9 Gy in 3 fractions [
20,
21]. They treated 72 patients with Stage I or II operable breast cancer with KORTUC, with or without chemotherapy and hormonal therapy. During a mean follow-up period of 51 months, they found only 1 local recurrence; another patient developed bone metastases. Disease-free survival and local control rates were both 97.1% at 5 years.
Following the study of Ogawa et al. [
19,
20,
21], several groups used the intratumoral hydrogen peroxide radiosensitization method to treat breast cancer [
6,
7,
8,
23,
24].
Table 1 summarizes the results reported so far. Subsequent investigators treated more advanced cases, and the treatment outcomes were not as good as those of Ogawa et al. Shimbo et al. [
23] treated 30 patients with locally advanced or recurrent breast cancer employing the KORTUC method, and the 3-year local control rate was 75%; the 2-year progression-free survival rate was only 24%. Obata et al. [
24] treated 5 patients with Stage I breast cancer with KORTUC, and no local recurrences or distant metastases have been observed during a median follow-up period of 65 months (range, 47–91 months). They also treated 2 Stage II patients with axillary lymph node metastases: one developed a local recurrence at 12 months, and another developed brain metastasis at 35 months and died at 56 months (personal communication, December 2022). In addition, Obata et al. [
24] treated 32 patients with Stage III or IV breast cancer, and a complete or partial response was obtained in 50%. So, the treatment efficacy depends on the disease stage.
Table 1. Studies on radiosensitization with intratumoral hydrogen peroxide injection for breast cancer.
This radiosensitization method with intratumoral hydrogen peroxide injection has spread to the United Kingdom, and a Phase I study was conducted for locally advanced breast cancer [
8]. Twelve patients were treated, and all had acceptable toxicity. At the last imaging assessment, the percentage of tumor volume reduction was between 50 and 100%. A Phase II study is now being conducted.
2.4. Whole-Breast Radiotherapy Followed by Stereotactic or Intensity-Modulated Boost with or without Radiosensitization Strategy
The authors’ group has been using conventional whole-breast radiation, followed by stereotactic or IMRT boost, for operable breast cancer patients who refuse any type of surgery. Details of the treatment have been described [
6,
7]; updated results are shown in this article. Until recently, we used 50 Gy in 25 daily fractions for whole-breast treatment, but currently moderate hypofractionation with 44.8 Gy in 16 fractions (2.8 Gy daily) is used. Standard boost doses were 21 Gy in 3 fractions for stereotactic irradiation and 20 Gy in 8 fractions for IMRT. The planning target volume for the boost treatment was the internal target volume plus 5 mm margins in all directions. The IMRT boost dose has recently been modified to 19.6 Gy in 7 fractions (2.8 Gy daily). For tumors approximately ≥2 cm in maximum diameter, two types of radiosensitization methods have been applied: one is hydrogen peroxide injection (KORTUC) during whole-breast radiotherapy, and the other is hyperthermia plus oral tegafur-gimeracil-oteracil potassium (S-1). The former radiosensitization method was terminated due to a change in medical legislation in Japan, and thereafter, the latter sensitization method has been employed. The hydrogen peroxide radiosensitization method is the same as that used by Ogawa et al. [
19,
20,
21]. Radiofrequency hyperthermia was performed with RF-8 (Yamamoto Vinitor, Osaka, Japan) once a week during radiation therapy up to five times. The skin temperature was maintained at 40–41.5 °C for at least 30 min. S-1 (80–120 mg/day) was orally administered twice a day from the evening before the starting day (usually Monday) of irradiation to the morning of weekends (usually Friday) and repeated until the treatment end.
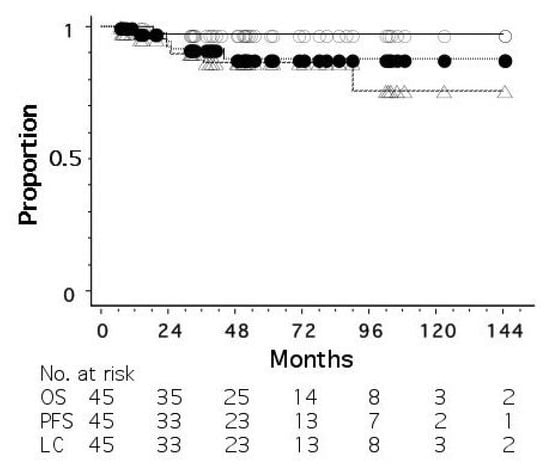
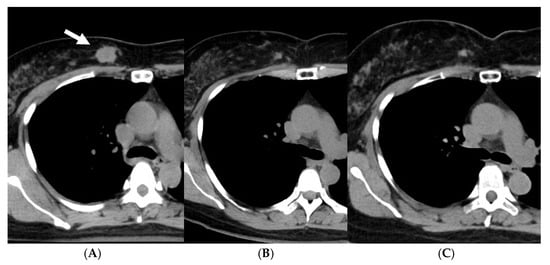
As of June 2022, 45 patients had been treated. The disease stages were 0 (ductal carcinoma in situ, DCIS) in 7 patients, I in 16, II in 19, and III in 3. The patients with a biopsy result of DCIS were staged as 0, but it was unknown whether the biopsy result represented the whole tumor. All the patients were judged to be operable by breast surgeons. Standard chemotherapy and/or hormonal therapy was used when the patients agreed to receive them; 4 patients received systemic chemotherapy and/or anti-HER2 therapy, and 31 of 35 hormone-receptor-positive patients received adjuvant hormonal therapy. Figure 1 shows overall, progression-free, and local recurrence-free survival curves for all 45 patients. The median follow-up period was 50 months (range, 6–180). The 5-year overall, progression-free, and local recurrence-free survival rates were 97.3, 86.4, and 87.9%, respectively. An important finding in our study is that even when a residual mass persisted after radiotherapy, the mass did not necessarily show regrowth. Figure 2 shows the changes of breast cancer over time. The original tumor achieved a partial response, but has remained stable after 22 months since treatment. We consider that these residual masses are usually scars, while fibroadenoma was detected by biopsy after 3 years of this treatment in a patient. Overall, 24 of the 45 patients had such a residual mass, but 20 of them have not developed local recurrence.
Figure 1. Overall (○), progression-free (△), and local recurrence-free (●) survival curves for 45 patients with operable breast cancer treated by whole-breast radiotherapy and stereotactic or intensity-modulated boost.
Figure 2. A case with an invasive ductal carcinoma (arrow) and a residual mass after treatment with whole-breast radiotherapy and stereotactic boost. (A) Before, (B) 22 months after, and (C) 81 months after treatment.
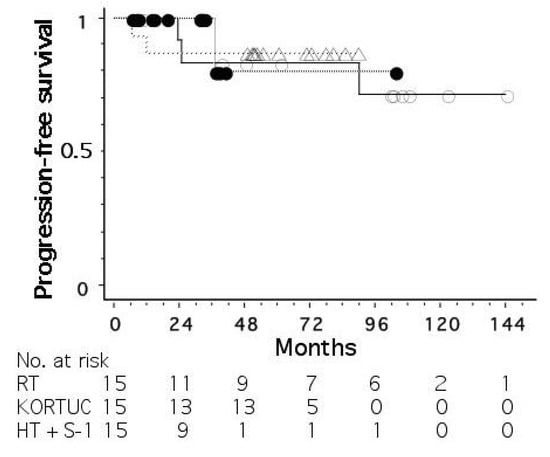
Figure 3 shows progression-free survival curves for the three groups treated without radiosensitization, with hydrogen peroxide sensitization, and with hyperthermia plus S-1 sensitization. The mean tumor size (±standard deviation) was 18 ± 11, 26 ± 9, and 27 ± 11 mm for the three groups, respectively. The stage distribution (0/I/II/III) was 2/9/3/1, 2/2/10/1, and 3/5/6/1, respectively. The 5-year progression-free survival rates were 83, 87, and 80%, respectively, for the three groups, with no significant differences among the groups.
Figure 3. Progression-free survival curves for operable breast cancer patients treated with radiotherapy alone (○), radiotherapy plus hydrogen peroxide radiosensitization (△), and radiotherapy plus hyperthermia and oral tegafur-gimeracil-oteracil potassium (S-1) (●).
Major toxicities were acute skin toxicities (radiation dermatitis), with Grade 1 in 23, Grade 2 in 7, and Grade 3 in 15. The Harvard Scale of breast cosmesis was excellent (nearly identical to an untreated breast) in 20, good (slightly different from an untreated breast) in 24, and fair (clearly different from an untreated breast but not seriously distorted) in 1. We reported enlargement of the irradiated breast probably due to lymph edema; although the enlarged breast is esthetically favorable, this produced asymmetry of the breasts, making the Harvard Scale “good” instead of “excellent”.
2.5. Particle Therapy
The use of proton or carbon ion beams has been considered for postoperative radiotherapy of breast cancer or for locally advanced cases [
25,
26]. Using proton beams in an anterior direction, irradiation to the lung can be minimized, and so it is expected that radiation pneumonitis would become almost zero. Attempts to use protons for definitive treatment of operable breast cancer are ongoing at two Japanese facilities of proton therapy. To our knowledge, however, patient accrual is limited, and no meaningful data are available at present.
Prospective studies of carbon ion therapy for Stage I breast cancer have been conducted. Results of a Phase I study, in which seven patients were enrolled, were reported [
27]. They received hypofractionated carbon ion therapy with 48, 52.8, or 60 GyRBE in 4 fractions. At 3 months after carbon ion therapy, 1 achieved a complete response, 5 achieved a partial response, and 1 had stable disease. The tumors were excised at 3 months after the treatment and were histologically evaluated; among the 7 patients, only 2 had a Grade 3 pathological effect. The conclusion of the study was that the timing of the histological evaluation (at 3 months) may not have been optimal.
Subsequently, the study entered Phase II, and three studies have been conducted [
28]. Although respective studies have limited patient numbers and are still ongoing, no local recurrence has been observed [
28]. The most recent study employed a single fraction treatment with 42–50 GyRBE using partial-breast irradiation. In addition, the results for 14 off-protocol patients undergoing carbon ion therapy for Stage I (T1N0M0) breast cancer were reported [
29]. Accelerated partial-breast irradiation was employed, and the radiation dose was 52.8 or 60 GyRBE in 4 fractions. Possibly due to the use of relatively high doses, 13 patients maintained a complete response, whereas only 1 patient developed local recurrence; this patient died of the disease at 69 months after carbon ion radiotherapy, while the other 13 patients were alive at 51–87 months (median, 61 months). Thus, particle therapy has not yet been established as a definitive treatment of early breast cancer, but carbon ion therapy may be worthy of further investigation.