Breast cancer is the most common malignancy in women worldwide. The cause of cancer is multifactorial. An early diagnosis and the appropriate treatment of cancer can improve the chances of survival. Recent studies have shown that breast cancer is influenced by the microbiota. Different microbial signatures have been identified in the breast microbiota, which have different patterns depending on the stage and biological subgroups. The human digestive system contains approximately 100 trillion bacteria. The gut microbiota is an emerging field of research that is associated with specific biological processes in many diseases, including cardiovascular disease, obesity, diabetes, brain disease, rheumatoid arthritis, and cancer.
1. Microbiome and Breast Cancer, the Connection
According to a 2018 global study, 13% of global cancer burden can be attributed to microbial infections, including both bacterial and viral infection, and this shows a clear geographical association [
19]. While the causative agents of cancer were determined to be
H. pylori, Human papilloma virus, Hepatitis B virus (HBV) and Hepatitis C virus (HCV) [
19], it should be noted that the human microbiome is composed of 10–100 trillion microbial partners, most of which remain unidentified. The highest rate of infection associated with cancer development was identified as being in eastern Asia, followed by sub-Saharan Africa, and then by northern Europe and west Asia. China alone accounted for one-third of the cancers driven by
H. pylori and human papilloma viruses [
19]. Therefore, the role of microbes in cancer requires increased attention. Breast cancer is not a single disease, but a variety of different cancers all affecting the breast. Molecular subtyping based on the presence or absence of cell surface receptors, such as ER, PR and Her2, therefore, is used to determine the correct treatment strategy. Patients lacking all three receptors or markers, or triple-negative breast cancer (TNBC) patients, have many adverse outcomes, due to lack of targeted therapies. Breast cancer is a multifactorial disease, potentially impacted by age, lifestyle, parity, exposure to carcinogens and genetics. However, 70% of breast cancers are detected without any known risk factors, other than those of being a woman and being above 50 years of age. Owing to advanced early detection, modern treatment strategies and the public awareness of breast cancer incidence has increased for the past four decades, by 0.5% annually, while mortality has dramatically declined [
20]. However, there is an inconsistency in predicting outcomes, particularly among younger and socially disadvantaged women, suggesting that other factors are at play. With the advent of modern sequencing technologies and multicentric techniques, the microbiota has emerged as a potential determinant of breast cancer severity and mortality. Breast cancer can be affected by the local breast microbiota or gut microbiota, either positively or negatively. The microbiota can increase/decrease the risk of breast cancer by regulating circulating steroid hormone levels, regulating energy intake and utilization, synthesizing metabolites, such as genotoxins, lipopolysaccharides, vitamins, and antibiotics, and modulating the immune system.
2. The Microbiota of Breast and Breast Tumor
The upsurge in human microbiome research was the direct consequence of the findings of the human microbiome project (HMP), which was initiated in 2007. By 2016, the microbiota of five different body sites of 300 healthy individuals, including the nasal cavity, oral cavity, skin, gastrointestinal tract, and urinogenital tract, was characterized and made publicly available. This marked the beginning of the second phase of the HMP, the integrated HMP (iHMP), which focuses on three non-infectious health conditions, pregnancy and pre-term birth, onset of inflammatory bowel disease and onset of Type 2 diabetes [
21]. The breast was considered sterile until Urbaniak et al. proposed the presence of a distinct microbial population in the breast that persisted beyond lactation [
22]. Eventually they and other groups, using deep sequencing techniques, such as 16s rRNA sequencing and shotgun sequencing, proved that there is indeed a microbial community living in breast tissue that is significantly altered in breast cancer [
23,
24]. Moreover, differences were evident between malignant and benign breast cancers and also between different subtypes of breast cancers [
24]. Several bacterial genera have been significantly associated with breast cancer. Tzeng, A. et al. examined the 16S rRNA gene sequence of human breast tissues compared to controls. A distinct microbial profile was associated with each histologic tumor subtype; for example, invasive ductal carcinoma (IDC) was characterized by the presence of
Tepidiphilus,
Alkanindiges, and
Stenotrophomonas, while samples of invasive lobular carcinomas (ILC) contained
Peptostreptococcus,
Micromonospora,
Faecalibacterium, and
Stenotrophomonas [
25]. However, their bioinformatic analysis showed that
Porphyromonas,
Lacibacter,
Ezakiella, and
Fusobacterium were more abundant at a more advanced stage than in lower-stage tumors [
25].
Parhi et al. showed that an oral pathogen,
Fusobacterium nucleatum, could translocate via the blood stream and accumulate in breast tumors, progressively increasing with stages of breast cancer [
26].
F. nucleatum has been shown to promote breast tumor growth and metastatic progression, possibly by preventing the accumulation of tumor-infiltrating T cells in the tumor microenvironment and colonizing breast tumors through D-galactose-β(1–3)-N-acetyl -D-galactosamine (Gal -GalNAc) which binds to Fap2, a surface lectin from
F. nucleatum, involved in the colonization of breast cancer. Furthermore, antibiotic therapy with metronidazole suppresses
F. nucleatum-induced breast tumor aggravation, indicating that targeting
F. nucleatum may enhance breast cancer treatment [
27]. In another detailed study, Parida et al. reported that toxin-producing strains of
Bacteroides fragilis, when present in the gut or breast tissue, could increase the aggressiveness of breast cancers, induce self-renewal in breast cancer cells and initiate metastatic dissemination to distant organs [
10].
The oral administration of
Lactobacillus acidophilus results in anti-cancer activity in mice bearing breast tumors, via stimulating the Th1 response and enhancing cellular immunity [
28]. Another study showed that
Lactobacillus helveticus R389 increased IL-10 and decreased IL-6 levels in serum and mammary cells, thereby suppressing mammary tumor cells by activating the local immune response [
29]. Oral administration of
Lactobacillus casei significantly increased the production of IL-12 and IFN-γ, thereby improving the immune response in mice with invasive ductal carcinoma [
30]. In addition, a population-based case–control study showed that the long-term exposure to probiotics, such as
Lactobacillus casei Shirota and soy isoflavones, protected against breast cancer in Japanese women [
31].
Improved imaging techniques, such as fluorescent in situ hybridization and modified PCR protocols, have allowed for the visualization of the spatial organization of microbial riders within the tumor, offering a sneak peek into their potential functions in shaping the tumor microenvironment. In a multicenter study of 1526 tumors and their adjacent normal tissues, Nejman et al. examined nine tumor types, including those in the breast, lung, ovary, pancreas, melanoma, bone, and brain. They demonstrated that tumor-specific microbes resided within tumors, as well as immune cells, in a cell-wall-deficient intracellular state [
32]. In addition, breast tumors were found to be the richest and most biodiverse among the nine tumor types examined. In another seminal study, Cai et al. proposed that the internal tumor environment enhanced the metastatic dissemination of breast cancers. The intra-tumor bacteria induce cytoskeletal remodeling in circulating breast cancer cells, making them more resistant to the fluid sheer stress in the circulation, thereby helping them establish colonies in distant sites [
33].
3. Gut Microbiome and Breast Cancer
A healthy human gut harbors between 300 and 500 bacterial species, predominantly composed of members belonging to four phyla, Actinobacteria, Bacteroidetes, Proteobacteria and Firmicutes [
34]. The most important physiological functions, energy assimilation, immune regulation, and xenobiotic metabolism, take place in the gut, and are largely accomplished by the gut microbes [
35]. In the context of breast cancer, the gut microbiota plays a complex yet crucial role. In addition to producing pro-carcinogenic toxins, such as BFT from
B. fragilis and colibactin from pks+
E. coli, which can potentially reach the breast tissue via circulation, gut microbes produce metabolites such as cadaverine [
36], indoxusulfate [
37], and lithocolic acid [
38], which are touted to hinder breast cancer progression.
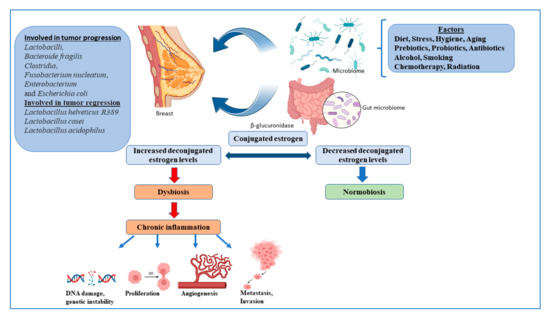
Multiple strains of the gut microbes are known to synthesize enzymes that deconjugate conjugated estrogen metabolites, preventing their excretion, and thereby regulating the levels of active estrogens in the circulation, one of the major promoters of breast cancer [
39]. Many bacterial species are also known to synthesize estrogen mimics e.g., seasmin, eterolactone and enterodiol, by breaking down dietary lignans [
39]. Gut microbial beta glucuronidases convert conjugated estrogen to deconjugated estrogen, which regulates breast dysbiosis, and leads to chronic inflammation, resulting in the alteration of the DNA breaks, proliferation, angiogenesis, metastasis, and invasion (
Figure 1).
Figure 1. The microbiome and regulation of estrogen in the breast cancer. Figures created with
BioRender.com.
Gut bacteria act through pathogen-associated molecular patterns (PAMPs), which regulate Toll-like receptors (TLRs) that are responsible for host defense against invading pathogens, and that activate signaling pathways that lead to the induction of immune and inflammatory genes. PAMPs are also responsible for inducing T cells, B cells and CD4 T cells to differentiate into Treg and Th17 cells, which return to the gut or enter the systemic circulation, which can affect immunity at different levels [
40]. The gut microbiota supports digestion, metabolism, and host immune responses, resulting in a symbiotic relationship between the host and microbiota, called the normobiosis, that maintains homeostasis [
41]. Dysbiosis is caused by changes in the microbiome, leading to a decrease in microbial diversity. As a result, the inability of the microbiota to defend against pathogenic organisms ultimately leads to local and systemic diseases [
41]. Obesity, an important breast cancer risk factor, is also closely associated with gut dysbiosis. Multiple studies, to date, have shown significant differences between the gut microbiota of healthy women compared to women with breast cancer, with some showing an overlap with obese microbiota [
23,
42]. Finally, multiple studies have shown that a healthy gut microbiota is indispensable for the effective utilization of drugs, chemotherapeutics, immunotherapy, and even radiotherapy [
40,
43].
This entry is adapted from the peer-reviewed paper 10.3390/pathogens12030468