Background: When a partial liver graft is transplanted into a recipient with portal hypertension, it is subject to sinusoidal shear stress, which, in good measure, is essential for regeneration. However, portal hyperperfusion which exceeds the capacity of the graft results in the small-for-size syndrome manifested by ascites, cholestasis and coagulopathy. This review discusses intraoperative hemodynamic variables that have been described in the literature, and inflow modulation strategies and their outcomes. Apart from using donor grafts which are of adequate size for the recipient weight, portal hemodynamics are an important consideration to prevent early allograft dysfunction, graft failure and mortality.
Summary: Understanding normal portal hemodynamics, how they change with the progression of cirrhosis, portal hypertension and changes after the implantation of a partial liver graft is key to managing patients with living-donor liver transplantation. If the intraoperative measurement of portal flow or pressure suggests graft portal hyperperfusion, inflow modulation strategies can be adopted. Splenic artery ligation, splenectomy and hemiportocaval shunts are well described in the literature. The proper selection of a donor to match the recipient’s anatomic, metabolic and hemodynamic environment and deciding which modulation strategy to use in which patient is an exercise in sound clinical judgement.
Key message: The intraoperative assessment of portal hemodynamics in living-donor liver transplant should be standard practice. Inflow modulation in properly selected patients offers a point-of-care solution to alter portal inflow to the graft with a view to improve recipient outcomes. In patients with small (anatomically/metabolically) grafts, using inflow modulation can result in outcomes equivalent to those in patients in whom larger grafts are used.
1. Normal Splanchnic Hemodynamics
The liver is uniquely placed to receive blood from the gastrointestinal tract through the portomesenteric system and is a powerhouse of molecular metabolic activity acting through this gut liver axis. From a purely mechanistic point of view, it serves as a reservoir that returns blood from the abdominal organs to the heart. The liver receives around 1/4th of the cardiac output while constituting only 2.5% of the body weight [
1], which amounts to 100–130 mL/min per 100 g of liver weight [
2]. The portal vein delivers around 70–75% of the blood, carrying 50–70% of the oxygen requirement, while the hepatic artery supplies the rest [
3,
4]. Liver sinusoids hold 60% of the blood, while the capacitance vessels (hepatic artery, portal vein and hepatic veins) account for the remaining 40% [
2]. Portal flow depends on the resistance offered by the liver bed as well as splanchnic and mesenteric arteriolar vascular tone. The lack of valves in the portal system helps maintain low pressure and low resistance. The normal range of portal venous pressure (PVP) ranges from 5 to 10 mmHg [
5]. As blood flows through the liver towards the heart, the pressure head drops progressively. Sinusoidal pressure or hepatic venous wedge pressure (3 to 10 mmHg) is between the PVP (5–10 mmHg) and inferior vena cava pressure (1–2 mmHg) [
6].
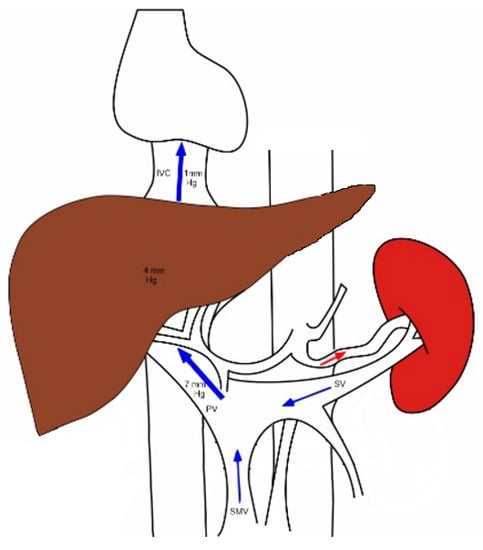
Figure 1 depicts the normal portal systemic circulation.
Figure 1. The major inflow to the liver comes from the portal vein, which receives blood from the spleen and the intestines via the splenic and mesenteric veins, respectively. The portal blood passes through a falling pressure gradient across the liver and reaches the systemic circulation via the hepatic veins into the right atrium. SMV—superior mesenteric vein; SV—splenic vein; PV—portal vein; IVC—inferior vena cava.
2. Changes That Occur in Splanchnic and Systemic Circulation in Chronic Liver Disease with Portal Hypertension
Portal hypertension is defined as a sustained mean pressure greater than 12 mmHg in the portal vein and its collaterals, which constitutes an increased risk for variceal bleeding and other complications [
7]. Clinically significant portal hypertension is defined as hepatic venous pressure gradient (HVPG) > 10 mmHg. This is associated with a significantly higher risk of decompensation and mortality [
8]. With the progression of liver parenchymal disease, the resistance to hepatopetal portal blood flow increases. The stellate cells lose their normal orientation and morphologically turn into myofibroblast-like cells [
9], and the sinusoidal endothelial cells become capillarized [
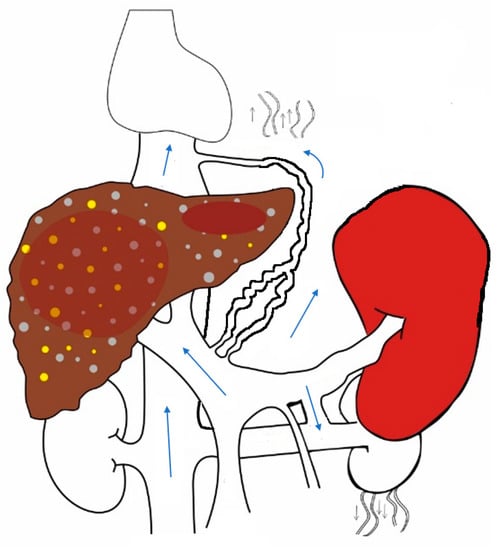
10], thereby leading to the disappearance of the sieve-plate structure. Multiple collateral channels open up and divert blood away from the liver. (
Figure 2) These portosystemic shunts result in splanchnic vasodilatation, and a hyperdynamic state ensues with high cardiac output and low systemic vascular resistance. Sodium and water retention occurs in response to this, with the expansion of plasma volume [
11,
12]. The liver and spleen, being solid organs, serve as compliance reservoirs while regulating mesenteric blood flow into the heart. There is a reciprocal relationship between hepatic and splenic sizes/volumes as blood flow is redistributed through portosystemic shunts.
Figure 2. In liver cirrhosis, sinusoidal resistance to portal flow increases and portal pressure increases, resulting in splenomegaly and the opening up of multiple portosystemic collaterals. Portomesenteric blood bypasses the liver and reaches the systemic circulation through these abnormal channels.
3. The Hepatic-Artery-Buffer Response
The relationship between the portal and arterial blood flow to the liver is regulated by local paracrine mechanisms by adenosine. This is known as the hepatic-artery-buffer response (HABR), which was first described by Lautt [
13]. Whenever portal flow increases, there is a corresponding decrease in hepatic artery flow. Adenosine, a vasodilator, is washed away from the sinusoids, and this leads to vasoconstriction and a decrease in arterial flow. Portal flow does not change reciprocally with changes in arterial flow. Portal flow in normal individuals is around 1500 mL/min, arterial flow is 300–400 mL/min and the ratio of portal to arterial flow is 2.5–3.5. With portal hypertension, in addition to the increase in portal flow, a decrease in arterial flow is also believed to contribute to graft dysfunction and ischemia to cholangiocytes. Post transplantation, partial liver grafts receive less hepatic artery flow compared to full grafts in absolute terms. However, the median hepatic arterial blood flow to the graft per 100 g of liver is not different in full or partial grafts. The ratio of portal-to-hepatic arterial flow increases from 6.6 to 15.4 post reperfusion in full and partial grafts, respectively [
14].
4. Changes That Occur When a New Liver Is Transplanted into the Hyperdynamic Circuit
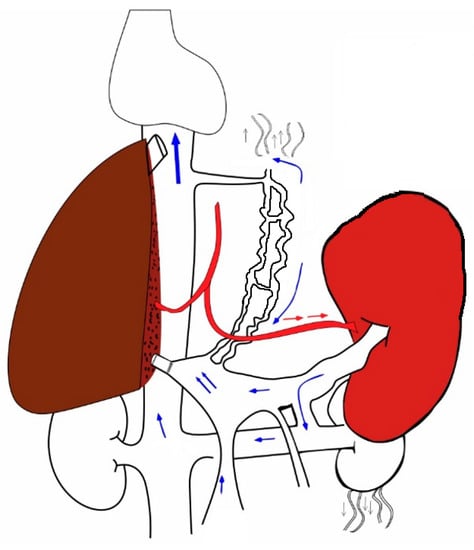
The relationship between pressure and flow is governed by the following equation. Portal Flow = Pressure gradient/Resistance. Resistance comes from the graft (both size and quality); flow and pressure are determined by the size of portal vein and extent of collateralization. The net flow per unit weight of liver depends upon the patency and the diameter of the portal vein, which is, in turn, inversely proportional to the extent and size of the portosystemic shunts. A sudden change in the pathologically altered hemodynamics by replacing the cirrhotic liver with a pliable donor liver results in a low-resistance pathway for the influx of a large amount of blood from the splenic vein (
Figure 3). The compliance of the graft liver is higher than that of the cirrhotic liver, and, hence, resistance to the portal flow is much lower. Therefore, the volume of blood flowing through a unit gram of liver tissue is higher. The shear stress of the blood flowing through the sinusoids is the trigger for hepatic regeneration. Periportal hepatocytes come in contact with heptotrophic growth factors with an increase in sinusoidal permeability [
15]. The portal flow should be optimal; too little flow hampers regeneration and graft function, and too much flow results in the all-too-well-known small-for-size syndrome (SFSS).
Table 1 summarizes the many definitions of SFSS which have been proposed by different groups [
16,
17,
18,
19,
20,
21]. In 2015, Dahm et al. [
18] proposed a definition of SFS dysfunction in a small partial liver graft (GRWR < 0.8) as the presence of two criteria (bilirubin > 100 umol/L, INR > 2, grade 3/4 encephalopathy) on three consecutive days in the first postoperative week after the exclusion of technical (arterial/portal occlusion, outflow congestion, bile leak), immunological (rejection) or infectious (cholangitis, sepsis) causes. Subsequently Hernandez-Alejandro et al. proposed a more comprehensive definition including portal flow (>250 mL/min/100 g liver) as a prerequisite in addition to size (GRWR < 0.8) [
20]. They considered SFSS to be present if two of the four parameters (ascites, hyperbilirubinemia, prolonged INR, hepatic encephalopathy) were present (
Table 1) in the absence of technical/immunological or infectious causes. The inclusion of portal flow marks the shift in understanding from size alone to size and flow paradigm in the pathogenesis of SFSS.
Figure 3. Depicts a right-hemiliver graft transplanted into a hyperdynamic circuit due to portal hypertension. The partial graft receives blood from the enlarged spleen.
Table 1. Definitions for small-for-size syndrome after liver transplantation.
Optimal portal flow is critical for liver regeneration. In a study of 64 recipients of right-hemiliver grafts who (all with a graft-recipient-weight ratio [GRWR] > 0.8) had an uneventful postoperative course, patients with an initial portal venous pressure of 23 mmHg and postreperfusion pressure of 15 mmHg had the best regeneration after 3 months of transplantation [
22]. The portal flows positively correlated with hepatic regeneration 2 weeks after transplantation [
23]. Portal venous velocity in the early post-transplant period is an important factor in liver regeneration [
24]. Valdecasas et al. showed that portal flow in the recipient increased almost four-fold one hour after reperfusion; this was, however, associated with no adverse events, as all the 22 recipients in this series received right-hemiliver grafts with a median GRWR of more than 1 [
25]. Portal venous flow is an important determinant of patient and graft outcomes. If the portal venous flow post reperfusion is more than four times the flow rate observed in donors (360 mL/min per 100 g), it is predictive of graft failure; flow rates less than half of that found in donors that resulted in poor survival [
14]. An elegantly conducted hemodynamic study in 28 recipients with cirrhosis who underwent orthotopic liver transplantation (OLT) evaluated parameters before transplant and after six monthly intervals for a mean follow-up period of 17 months. After OLT, most systemic hemodynamic parameters such as heart rate, mean arterial pressure, peripheral vascular resistance and cardiac index normalized. However, although spleen size decreased it continued to be larger than in controls and did not return to normal [
26].
A critical mass of liver which adapts to the new hemodynamic milieu and successfully hypertrophies to match the metabolic demand of the recipient is a sine qua non for good outcomes. A resetting of the flow and pressure systems occurs while the graft copes and functions in the new setting. The proper selection of donors, preoperative recipient evaluation, sound surgical technique, intraoperative management of recipient portal flow and pressures and diligent postoperative care contribute towards this.
5. Pharmacological Measures
An increase in hepatic resistance contributes to portal hypertension as cirrhosis progresses. At a later date, an increase in portal flow sustains portal hypertension. Initial clinical hypotheses of the mechanisms of portal hypertension were tested in animal models, and the molecular factors involved were elucidated [
72]. Pharmacological measures that reduce portal pressure but preserve HABR have a favorable effect on graft function. Mehrabi et al. investigated the role of systemically administered vasopressors (epinephrine and norepinephrine) and found that both reduced PVF and hepatic arterial flow in porcine liver transplantation [
73]. Vasopressin and Terlipressin have a selective effect on portal pressure due to their action on V1a receptors [
74]. Wagener et al. [
75], in a study of 16 patients, found that Vasopressin decreased portal pressure and flow by causing splanchnic vasoconstriction, without affecting cardiac output or mean arterial pressure. In a double-blind randomized controlled trial of the routine perioperative use of Terlipressin in adult LDLT by Reddy MS et al., authors did not find any reduction in postreperfusion portal pressure with the systemic use of Terlipressin [
76]. However, Terlipressin infusion reduced ascites formation and, therefore, the need for paracentesis. The length of hospital stay was lower in the Terlipressin group. Due to possibility of side effects such as a rise in lactate level and symptomatic bradycardia, Terlipressin should be used with close monitoring in patients with high-volume ascites. In a rat-liver transplantation model, the use of low-dose Somatostatin reduced graft injury, postulated to be due to a reduction in shear stress due to increased portal flow [
77]. Granulocyte-colony-stimulating factor [
78] and hyperbaric oxygen treatment [
79] have been shown to reduce liver injury in a massive hepatectomy model in rats. In study by Suehiro T et al., authors continuously administered intraportal Nafamostat mesilate, a Prostaglandin E1 and Thromboxane A2 synthetase inhibitor, by double-lumen catheters for 7 days [
80]. This reduced hyperbilirubinemia and ascites in patients with small-for-size grafts. There are also case reports in which propranolol, alone or along with somatostatin, was used to treat SFSS that occurred despite GIM [
81,
82]. A randomized trial by Troisi et al. found that somatostatin infusion reduced HVPG while preserving arterial inflow to the graft [
83]. Pharmacological modulation has the potential to augment the effects of surgical modulation; this needs further study. Interventions to improve hepatic artery flow in small-for-size livers is another strategy. Kelly DM, Zhu X et al. demonstrated improved survival with the infusion of adenosine into the hepatic artery in an animal model [
84]. Currently, these methods are not used widely.
This entry is adapted from the peer-reviewed paper 10.3390/transplantology4020006