Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Oncology
Trophoblast cell surface antigen-2 (Trop-2) is a glycoprotein that was first described as a membrane marker of trophoblast cells and was associated with regenerative abilities. Trop-2 overexpression was also described in several tumour types. Nevertheless, the therapeutic potential of Trop-2 was widely recognized and clinical studies with drug–antibody conjugates have been initiated in various cancer types.
- Trop-2
- effect
- cancer
1. Background
1.1. TROP-2 Biology and Functions
The transmembrane glycoprotein trophoblast cell surface antigen-2 (Trop-2) is widely expressed in various epithelial cancers as well as in specific normal tissue. Trop-2 is also known as tumour-associated calcium signal transducer 2 (TACSTD2), membrane component chromosome 1 surface marker 1 (M1S1), gastrointestinal antigen 733-1 (GA733-1), and epithelial glycoprotein-1 (EGP-1) [1].
Trop-2 was initially discovered in placental trophoblastic tissue, and the cells expressing this biomarker have the capacity to invade the uterus during placental implantation [2,3]. Lipinski et al. identified four new transmembrane glycoproteins (Trop-1, 2, 3, and 4) expressed on normal and malignant embryonal cells and, among them, only Trop-2 may similarly confer the capacity for proliferation and invasion to cancer cells [4].
Although the physiological function of Trop-2 is not fully clarified and is still under investigation, Trop-2 is implicated in several intracellular axes, including the MAPK/PI3K/AKT pathways that are implicated in proliferation, migration, and invasion of cancer cells [5,6,7]. Overexpression of Trop-2 was associated with accelerated tumour growth and a dismal prognosis in various types of cancers, including breast, gastric, and ovarian cancers [8,9,10]. Conversely, in other tumours like non-small cell lung cancer (NSCLC), TROP-2 downregulation and internalization into the cytoplasm, are related to metastasis and recurrence [11]. Trop-2 is also overexpressed in haematologic malignancies like as leukaemia, extranodal nasal type lymphoma (ENK/TL), and non-Hodgkin’s lymphoma (NHL) [12,13].
These characteristics could make Trop-2 a seductive target for cancer therapy. Currently, numerous therapeutic strategies with antibodies or antibody–drug conjugates are being developed to target Trop-2 in specific tumours.
1.2. Trop-2 Properties, Binding Partners, and Signalling Pathways
Trop-2/TACSTD2 was first described in 1981 and its gene is located on chromosome 1p32 [2,4]. The tertiary structure of Trop-2 consists of multiple domains that extend through the cell membrane. The extracellular domain is composed of a 26-amino acid hydrophobic peptide and an N-terminal part, the largest part of the molecule consisting of 274 amino-acids, also known as the ectodomain (Trop-2EC). It is comprised of an epidermal growth factor-like repeat containing a cysteine-rich domain, a thyroglobulin type-1 domain, and a cysteine-poor domain, anchored via a single transmembrane helix (TM) followed by a short intracellular tail (Trop-2IC) [14].
The 30-amino acid cytoplasmic part shows high homology to a HIKE domain [15,16] and includes a serine residue (S303) that is phosphorylated by protein kinase C (PKC) [17] and a phosphatidyl-inositol 4,5-bisphosphate (PIP2) binding site [8].
Trop-2 is a member of a protein family (GA733 family) that includes at least two “type I” membrane proteins: GA733-1 (Trop-2) and GA733-2, also known as EpCAM (epithelial cell adhesion molecule). Trop-2 and EpCAM exhibit very high similarities in sequence and structure, with 49% homology and 65% similarity in amino acid repeats and a comparable arrangement of hydrophilic and hydrophobic parts [14,18]. Nevertheless, the promotor regions of EpCAM and Trop-2 are unrelated, resulting in different expression patterns [19] and leading to structural differences in the intracellular domain explaining the distinct intracellular signalling and functions between Trop-2 and EpCAM [20,21]. Indeed, EpCAM exhibits its role in cell differentiation, proliferation, and migration through c-myc. On the contrary, Trop-2 has been reported to interact with several proteins, such as insulin-like growth factor-1 (IGF-1) 11, claudin-1 and 7, cyclin D1, and PKC. Furthermore, due to the HIKE domain, the PIP2 binding site, and the serine phosphorylated by PKC, Trop-2 is involved in calcium signalling through which the MAPK pathway could be activated [5].
1.2.1. IGF-1/IGF-1R
IGF-1, as mentioned above, binds Trop-2 leading to modulation of IGF-1 signalling and activation through PIP2 and Ca2+. Trop-2 may also bind the receptor of IGR-1 (IGF-1R), blocking IGF-1 signalling [11] and playing critical roles in cell growth, differentiation, transformation, and metastasis. This mechanism could explain the different impacts of Trop-2 overexpression in lung cancer. Indeed, in a lung cancer model, high expression of Trop-2 suppressed tumour growth by attenuating IGF-1R signalling, likely by binding IGF-1 [14].
1.2.2. Claudin
Claudin-1 and 7, two transmembrane proteins composing the tight junctions at the epithelial surface, bind to Trop-2′s ectodomain preventing claudin degradation which plays a fundamental role in epithelial barrier maintenance. Trop-2 might also indirectly affect adhesive interactions between cells by modulating the complex formation between fibronectin and P1 integrin/RACK1 (receptor for activated PKC) [14].
1.2.3. ERK1/2
Trop-2 could also initiate the ERK1/2-MAPK axis, leading to malignant transformation [11], and could dysregulate stem cell function via the Notch, Hedgehog, and Wnt pathways through the expression and activation of cyclic AMP-responsive element-binding protein (CREB1), Jun, NF-κB, Rb, STAT1, and STAT3 (Figure 1) [5]. As previously mentioned, the MAPK pathway is stimulated by increased Ca2+ and phosphorylation of MAPK, which affects cell cycle progression. Furthermore, ERK activation was observed in various tumour types characterized by Trop-2 overexpression, and this ERK1/2 activation is thought to promote tumour survival through anti-apoptotic effects [5].
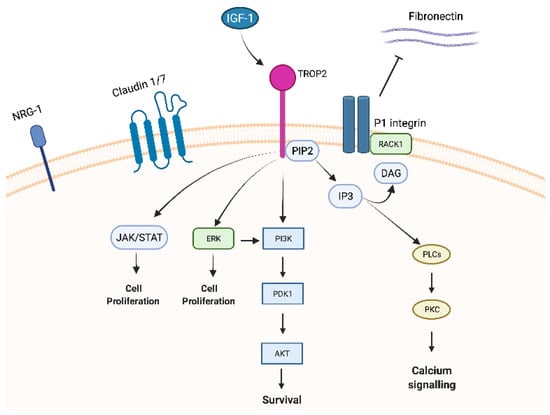
Figure 1. Trop-2 membrane-associated interacting partners and signalling pathways.
1.2.4. NRG1
Trop-2 seems to also directly affect NRG1 via the EGF-like and thyroglobulin repeat domains in the extracellular region of Trop-2. In a pre-clinical model of HNSCC cells, Zhang and colleagues knocked down Trop-2 resulting in increased membrane localization of NRG1 that binds to and increases the activation of ErbB3 [22].
1.2.5. Cyclin D1
Trop-2 could create a fusion product with cyclin D1 (bicistronic cyclin D1/Trop-2) [23] due to post transcriptional processes. This change could affect the stability of cyclin D1 increasing cell longevity and proliferation and qualifying Trop-2 as an oncogene.
1.2.6. PKC
Lastly, PKC phosphorylates Trop-2′s cytoplasmic tail at S303. PIP2 has been suggested as a phosphorylation regulator of S303 by PKC through activation of the Raf and NF-κB pathways, promoting cancer cell survival [24].
2. TROP-2 Significance in Cancer
Despite Trop-2 being first identified as a cell surface marker for trophoblast cells, a great effort has been made to elucidate the role of this marker in cancers [21].
Trop-2 was also detected in stem cells of different tissues, especially in basal cells. As an example, the expression of TROP- 2 had been associated with self-renewal, regeneration, and differentiation properties in prostate basal cells [25,26], oval cells after liver injury [27], and endometrium-regenerating cells [28]. These findings support the role of Trop-2 as a regulator of stem cell growth which is implicated in the regeneration of various tissues, perhaps playing a role in physiological events like hyperplasia.
On the other hand, as noted above with few exceptions, its overexpression has also been associated with the increase in tumour growth, proliferation, and metastasis in various epithelial cancers, i.e., head and neck, thyroid, lung, gastrointestinal tract, breast, renal, and gynaecological cancers, and glioma [29].
Beyond the prognostic value, the real weight of Trop-2 gain of function or Trop-2 loss in tumorigenesis, epithelial–mesenchymal transition (EMT), and mesenchymal trans-differentiation remains unclear [30,31].
Silencing the Trop-2 gene using small interfering (si) RNA in colon, breast, cervical, lung, and ovarian cancer cell models led to a suppression of malignant transformation inhibiting the proliferation, invasion, and the formation of colonies in vitro [30,32,33,34,35]. The knockdown of Trop-2 in gallbladder cancer inhibited vimentin and increased E-cadherin expression linked to EMT [36]. Trop-2 overexpression seems to be related to an increased risk of metastasis in patients affected by various cancer types (oral squamous, thyroid, some oesophageal, gastric, colorectal, pancreatic, ovarian, uterine, cervical, prostate, and urinary bladder). However, it is not upregulated in others (e.g., head and neck and certain lung cancers, such as lung adenosquamous and squamous cell carcinoma) [1,29,37,38,39].
In gastric cancer, the poor prognostic value seems to be related to the co-expression of Trop-2 and amphiregulin [40], while tumour necrosis factor-α (TNF-α) axes could regulate this effect in colorectal cancer. Indeed, a low concentration of this cytokine correlates with an increase in Trop-2 protein expression, while higher concentrations of TNF-α reduce migration and cell invasiveness [41]. These authors linked these activities to the crosstalk between TNF-α and the ERK1/2 pathway, showing that an ERK1/2 inhibitor can suppress the cytokine’s upregulation of Trop-2 [41]. The ERK1/2 pathway was also involved in pancreatic cancer, gynaecological cancers, and HNSCC, where Trop-2 expression increases the phosphorylation of ERK1/2 leading to the activation of the ERK/MAPK pathway, increasing the levels of cyclin D1 and cyclin E that resulted in a cell cycle dysregulation [5,32,42]. High cyclin D1 expression seems to also be the result of the activation of Trop-2 via in breast cancer [32,43].
In a prostatic model, Goldstein et al. showed that a malignant transformation could arise from the Trop-2+ basal cells in immunodeficient mice [26]. Thus, basal cells expressing Trop-2 and CD44 can develop luminal phenotype tumours [25,26,44,45]. This is consistent with studies implicating Trop-2 as a critical regulator of β1 integrin activities and promoting prostate cancer cell motility [3,46]. Interestingly, Trop-2+ exosomes purified from prostate cancer promote migration of Trop-2-negative prostate cancer cells on fibronectin, suggesting that Trop-2 could induce cells lacking Trop-2 to gain Trop-2 regulatory properties affecting migration [47].
The role of Trop-2 in haematological disease is still unclear, since it is expressed in Hodgkin’s lymphoma and chronic lymphocytic leukaemia1, but not in anaplastic large cell lymphoma [13].
To summarize, the Trop-2 gene is related to several transcription factors leading to a dysregulation of the numerous pathways connected with this glycoprotein. Although not yet fully elucidated, it activates CREB1, Jun, NF-κB, Rb, STAT1, and STAT3 via induction of the cyclin D1 and ERK/MEK pathways affecting malignant transformation and metastasis [8,33,48,49].
This entry is adapted from the peer-reviewed paper 10.3390/cancers15061744
This entry is offline, you can click here to edit this entry!
