Synaptosomes are subcellular components isolated from nerve terminations that can be prepared by homogenizing brain tissue in isotonic sucrose solution followed by appropriate centrifugation. Their preparation technique has a long history since synaptosomes were first isolated from nerve endings and described by Gray and Whittaker in 1962. The preparation of synaptosomes produces presynaptic boutons alone or in combination with fragments of postsynaptic membranes. Interestingly, synaptosomes contain organelles and vesicles that express native channels, receptors, and transporters. At 37 °C, these isolated nerve endings are metabolically active and synthesize and release neurotransmitters. They are actively used to investigate neurotransmission, its actors, and the mechanisms of neurotransmitter release. To date, many functional and non-functional applications of synaptosomes have been documented. Due to their versatility, synaptosomes have been actively used to study neuroinflammatory processes.
Neuroinflammation is a reaction that involves all cells present in the central nervous system (CNS), including neurons, macroglia, and microglia [
1,
2,
3,
4]. Immune responses can be beneficial or harmful to the brain, depending on the degree to which they are activated. Inflammation is mediated by the production of cytokines such as interleukin IL-1 and IL-6, TNF-α and TGF-β [
5], chemokines, reactive oxygen species, and second messengers [
6]. Neuro-inflammatory reactions have immunological, physiological, biochemical, and psychological effects [
2]. Additionally, neuroinflammation depends on several conditions (context, duration, and primary stimulus) [
7] and can be supported by mechanisms at the synaptic level [
8,
9,
10]. Accordingly, TNF-α is involved in an acute phase of inflammation but is also responsible for several physiological functions and supports synaptic connection [
1]. However, uncontrolled expression of TNF-α could lead to synaptic loss and glutamatergic toxicity through modulation of the glutamate receptor [
6]. Glutamate release and synaptic plasticity could be inhibited by IL-6 [
7]. Therefore, the study of synapses became an important task in several neurodegenerative diseases characterized by high levels of neuroinflammation [
11]. There are several models for studying synapses, but one that is largely used is synaptosomal preparation [
11,
12,
13,
14].
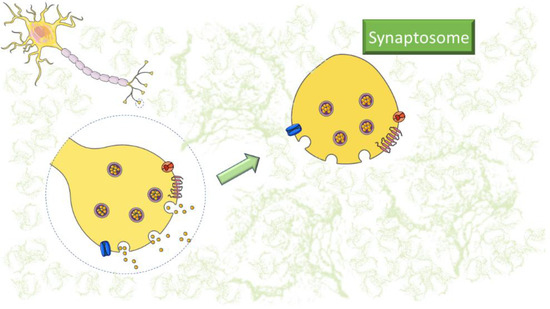
Synaptosomes are a convenient tool for neurochemical and electrophysiological studies due to the preservation of enzymatic and metabolic activities, as well as being an outstanding research tool for understanding the mechanisms of synaptic dysfunction [
15,
16,
17,
18,
19,
20] (
Figure 1). The contribution of ROS to synaptic dysfunction in AD pathogenesis was investigated through synaptosomes from cortices of APP/PS1 mice, observing an increase in septin-3, septin-5, and C1q accumulation [
21]. Consequently, an increase in SUMO-1ylation (small ubiquitin-like modifier), which was also present in human AD brains, was observed in cortical synaptosomes from Tg2576 mice. Marcelli and colleagues have shown that increased SUMO-1ylation contributes to the development of synaptic dysfunction in AD [
22]. Synaptosomes can be easily prepared using the standard procedure [
12]; once the tissue is collected, it is homogenized in 0.32 M sucrose and centrifuged. After the first centrifugation, the supernatant is further centrifuged to obtain the crude synaptosome pellet (P2) [
23,
24]. In case a higher grade of purity is requested, the samples could be obtained by density gradient (Percoll, Ficoll, or sucrose) centrifugation [
25,
26,
27,
28].
Figure 1. Representative image of synaptosomes. The figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license (
https://smart.servier.com/, accessed on 7 February 2023).
Furthermore, synaptosomes could be prepared from post-mortem human brain tissue and used to study disease pathogenesis and neurotransmitter synthesis and release in the brain of patients [
15,
29]. Several pathologies, such as Huntington’s disease (HD) [
30], Parkinson’s disease (PD) [
26,
31], Alzheimer’s disease (AD) [
32,
33,
34], and amyotrophic lateral sclerosis (ALS) [
35], have been studied monitoring synaptic dysfunction through synaptosomes [
36]. Additionally, synaptosomes have been observed to undergo functional changes upon acute exposure to receptor ligands/enzyme modulators in vitro. These changes persist after removing the triggering agents, but they can also adapt to and maintain the structure of the tissue of origin chronically exposed to chemicals [
37].
Using the entrapping technique, membrane-impermeant agents of different sizes present in the homogenization medium could be internalized for studying intracellular mechanisms [
38,
39,
40]. Recently, synaptosomes were described to release exosomes and extracellular vesicles able to participate in physiological and pathological neurotransmission [
41].
This “Entry” paper will briefly summarize the knowledge on the use of synaptosomes for studying neuroinflammation markers (Table 1). In particular, researchers will analyze methods applied to synaptosomes to highlight neuroinflammatory parameters considering mature acquisitions and recent publications.
Table 1. Summarized overview of the techniques, diseases, and neuroinflammation markers described in this entry.
| Analytical Technique |
Disease |
Neuroinflammation Markers |
References |
Western Blot
Flow Cytometry
Neurotransmitter release
Proteomics
Confocal microscopy |
Alzheimer’s disease |
ROS |
[1,5,11,21,29,32,42,43,44,45,46,47,48,49,50,51,52,53,54] |
| C1q |
| iNOS |
| TNF-α |
| IL-1β, IL-6, IL-1 |
| CCL2, CCL5, CXCL1 |
| Parkinson’s disease |
TNFα |
[1,26,49,55,56] |
| IL-6 |
| NOS2 |
| ROS |
| Huntington’s disease |
TNF-α |
[1,30,44,55] |
| IL-1β |
| IL-10 |
| NO |
| Epilepsy |
ROS |
[57,58] |
| iNOS |
| NMDA-induced excitotoxicity |
IL-1β, IL-6, TNFα, mPGES-1, COX-2 |
[55,57] |
| iNOS |
| SAE |
IFN-γ |
[59,60] |
| IL-1β |
| IL-10 |
| TNF-α |
| IL-6 |
| CXCL1 |
| Influenza A Virus |
CD80 |
[61] |
| CD36, CD68, |
| C1QA, C3 |
| HFD |
BDNF |
[62,63] |
| IL-1β |
| TNF-α |
| NF-κB |
| Multiple Sclerosis |
Iba-1 |
[64,65,66,67,68,69,70] |
| TNF-α |
| IL-17 |
| CCL5 |
| CXCR4 |
| Th1, Th17 |
| ROS |
| Amyotrophic Lateral Sclerosis |
IL-1β, IL-12, |
[35,71,72] |
| IFN-γ, IL-1α |
| C1q |
| ROS |
| COX-2 |
| iNOS |
| TNF-α |
This entry is adapted from the peer-reviewed paper 10.3390/encyclopedia3020027