Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Integrated pest management (IPM) is a promising technology for the environment. Insect pests and weeds have long posed a danger to rice production systems, resulting in severe output losses. Although insect, pest, and weed control has remained the most efficient plant protection tool, environmental risks have prompted scientists to propose alternate pest management options. The understanding of sustainable conventional agriculture prompted the broad deployment of IPM.
- biological control
- pest management
- rice
1. A New Era in Integrated Pest Management
Insect pests and diseases are the major problem for crops, fruits, and vegetables globally. Worldwide, different techniques have been adopted to control these insect pests and prevent yield losses. In the beginning, most people depended upon pesticides and insecticides, but later, due to their hazardous effect on health and the environment, alternative methods were adopted. These methods include biocontrol [1][2], resistance varieties [3], botanical extract [4][5][6][7][8], essential oils [9][10], volatile organic compounds that change preference and host selection behavior of insect pests [11][12][13],
The techniques used in insect pest control are described in Figure 1.
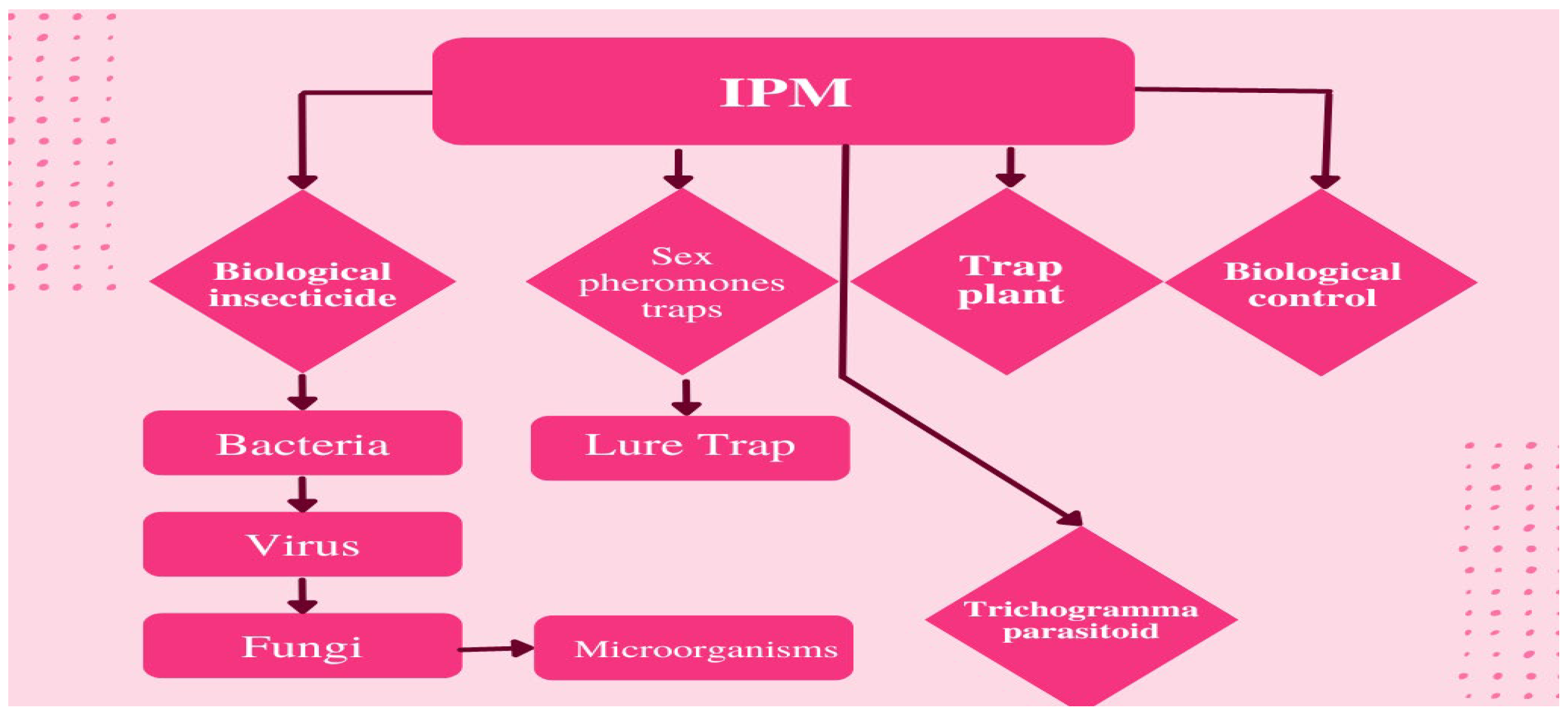
Figure 1. Integrated pest management techniques for rice.
1.1. Biological Insecticides
In China, as popular and governmental concern for the environment and food safety has increased, environmentally friendly biological pesticides have been more broadly approved and widely employed. Bt is China’s most popular biological insecticide for rice stem borers and RLFs and is officially recommended for use against rice stem borers. SSB and RLF effects are 65.31%, −96.69%, and 88.00–97.17%, respectively. After spraying the control effect of nuclear polyhedrose virus Mamestra brassicae on RLF, 14 d is more than 83%. More insecticidal rice pests, including RLF and SSB Lepidoptera, are controlled by Spinosad, Spinétoram, Bassian Beauvera, and BREVERS of Empedobacter. Cnaphalocrocis Medine Granulovirus (Cnme GV), which possesses an RLF synergy of Bt compounds, is another possible biological insecticide in the management of RLF. CnmeGV and Bt were the initial dead time for RLF treatment, 3 d short of the one treated only with CnmeGV, 20.23 percent mortality and more than 30% duration. Nine percent 12 THR biological insecticide is highly controllable in RLF, BPH, and WBPH [14][15][16][17][18].
1.2. Release Trichogramma Parasitoids
In China, many Trichogramma release studies were carried out in the 1950s to control Lepidoptera pesticides in rice paddy fields. Trichogramma parasitoids control SSB and RLF. However, Trichogramma parasitoids cannot be used in rice fields at large due to the problems of selection, cultivation, and application of field techniques, including Trichogramma. Trichogramma has just been recognized as meeting food, ecological, and environmental safety requirements for insect pest management in rice fields. T. japonicum, T. chilonis, T. dendrolimi, and T. ostriniae are the four most prevalent Trichogramma species discovered in rice fields [19]. T. dendrolimi thrives in temperatures between 18 and 26 degrees Celsius, while T. japonicum thrives in temperatures between 30 and 34 degrees Celsius. Meanwhile, after 4 days, Trichogramma is unable to parasitize any SSB egg successfully [20][21]. In the parasitization of RLF eggs in regions with any of the above two temperatures, particularly in regions with high temperatures, T. japonicum works best. There is no difference in parasitizing 1–3 d RLF eggs among all four Trichogramma species, but parasitism of the four-day-old eggs has declined significantly. In recent years, Trichogramma release technologies have also been developed, including the appropriate rice field release devices for Trichogramma, nectar-food supplement release instruments for Trichogramma, and unmanaged aircraft release techniques for Trichogramma [21][22][23]. A series of Trichogramma release technology demonstration tests were carried out by the China National Chinese Service for Extension of Agricultural Technology to improve Trichogramma’s application in rice insect pest control. These tests included species selection, time release intervals, times of application, height, and density. These experiments can potentially improve technological standardization while laying the groundwork for the widespread use of Trichogramma in paddy fields (unpublished data).
1.3. Sex Pheromones Cause Mate Conflict
SSB and RLF pheromones have been successfully developed and are easy to use. Only one sex pheromone should be utilized in each trap for maximum effectiveness, as the appealing capacity of two or more sex pheromones may be reduced. The ideal trap height is 10–20 cm beneath the rice canopy. The pest control effect of sex pheromone traps was shown to be greater than 50% in demonstration trials on a wide scale, and it may be further increased by combining it with other pest management tactics. When used to control SSB and RLF, sex pheromone traps can save up to two sprays of insecticide. In some cases, the pest control results of sex pheromone traps are comparable to those of insecticides, while the input costs are slightly cheaper [17][22][23][24][25].
Overwintering SSB has a 40–60-day emergence phase, and its oviposition phase is equally protracted. SSB sex pheromones, on the other hand, have an effective work time of more than 50 days, and a large number of male SSB adults can be trapped and killed (the maximum number of single traps is more than 130 individuals). The populations of the first-generation SSB in the field can be dramatically reduced by catching a significant number of males from the overwintered generation and significantly reducing the death rates induced by the first generation of SSB. For example, if more than 300 pheromone traps per hectare are placed in isolation and large regions, the number of SSB eggs is decreased by more than 70%. When there are 60 sex pheromone traps set up in large demonstration sites every hectare, the mass of eggs is reduced by over 70% both on rice seedlings and on rice grounds. The larger the area of sexual pheromone traps, the more effective the control of pests [26].
1.4. Application of Trap Plants
An effective conservation biological control method, trap cropping involves growing another non-crop in a designated area in order to attract pests from a target crop, prevent them from reaching the crop, and then control those pests in order to reduce damage to the crop. Trap cropping has been successfully used for managing various insect pests since the 1930s, resulting in a substantial reduction in the use of pesticides in developing countries. Due to the high population of pests on these new trap plants placed in agricultural fields, trap cropping must prevent insect dispersal back onto the focal crop to be effective. Borers of rice stems, such as SSBs and PSBs, lay their eggs on the vetiver grass Vetiveria zizanioides, but their life cycle is not complete. In rice fields, vetiver grass may effectively “drag” borers into mature rice stems for eggs, reducing pest levels. The vegetable plantation is determined to represent about 6–10% of the rice cultivation area between late March and early April. To further diminish the populations of stem borers, eggs and early larvae can be ripped down with certain treatments [26][27][28].
1.5. Animal Husbandry Model Farm
Since it has evolved through self-regulation, the combination of the ecological planting and breeding system, which includes a combination of rice ducks, rice fish, rice soft-shelled turtles, and rice crabs, is a perfect system in comparison to the traditional rice cultivation system.
Ducks are introduced into the rice–duck system to reduce inefficient tillers, encourage the exchange of gas, improve the decomposition of effective soil components, increase rice pest resistance, and reduce rice pests. For example, the fourth and fifth generations of rice cultivations fell by 70.2% and 70.4% in the mid-rice seasons, and by 56.2% and 64.6%, respectively, in the late rice seasons. Furthermore, SSB can effectively be controlled by mutual rice–duck behavior in paddy fields. The number of SSB larvae of the second and third generations has decreased by 53.2% to 76.8% and 61.8%, respectively. In mid-season rice and late-season rice, the SSB damage has been reduced by 13.4–47.1% [29][30][31].
Additionally, rice ducks can increase the number of natural enemies significantly and reduce pest populations. Compared to conventional paddy fields, rice cuckoo fields held 63.6 percent more spiders, and conventional paddy fields held 1.65–2.61 times more spiders [29]. In early and late season, ratios of spider hoppers to rice plants have been increased by 2.3 and 2.1 times, respectively, with ducks. RLF larvae are at a parasitic rate of 53.0–61.3% for duck-filled rice in the early season and the rate of rice with duck in the late season ranges from 29.4% to 38.3%. Compared to conventional rice farms, they are 1.05–3.21 times larger [32][33].
2. Integrated Pest Management
As efforts have been made to reduce the use of chemical controls in an attempt to reduce their impact on the environment and on human health, there has been an increase in the number of environmental and human health outcomes associated with the use of pesticides in recent decades. The development of alternative pesticides is undoubtedly needed, but how will they be developed? Insecticide treatments can be greatly reduced by eliminating rice core pests more effectively through non-insecticide control. Historically, by using chemical pesticides widely in the fight against major pests in field crops, new secondary pesticides (rice leaflets in Pakistan) have emerged by suppressing the activity of natural arthropods, these enemies normally disregard these secondary pests.
- ❖
-
Several individuals or combined tactic products are expected to reduce the use of insecticides in various agricultural products, not only for rice but across the board in the IPM. Improved scouting and monitoring and sophisticated, accurate crop simulations can easily lead to a 50 percent reduction in the accurate estimate of injury levels.
- ❖
-
Improved crop rotations, including amended laying methods and effective management of crop residues; lower and more efficient use of insecticides.
- ❖
-
Improved crop rotations, with modified techniques for tillage and effective management of crop residues.
- ❖
-
Improved crop types that are more resistant to pest attacks.
- ❖
-
Transgenic plants are included.
- ❖
-
Biological control is a term used to describe how something is controlled biologically.
- ❖
-
Natural enemies have a long history of reducing the impact of pests. Integrated pest management (IPM) can be achieved through inoculative increases, introduction and setup approaches, as well as introducing new pathogenic strains into existing species. Insect biological control is based on three basic approaches, increasing and conserving natural enemies, and insect pathogens. These approaches were effective, but they only received a fraction of the research on parasitoid and similar predatory approaches. These approaches minimize entomopathological weaknesses, including slow weakening of pests and populations, and take advantage of the recycling, persistence, and quick generation environmental forces. It is important to further refine and adapt the biological control approaches and application to realize the complete potential of this biologically based pest management strategy.
- ❖
-
The individuals responsible for implementing the IPM are the most important audience for the assessment results. These include farmers, agents of extension, and phytosanitary control. All efforts will be academic exercises without the members of this public recognizing and building upon the central and immune economic influence of biological control in their decision making.
This entry is adapted from the peer-reviewed paper 10.3390/su15054499
References
- Fu, W.; Yu, X.; Ahmed, N.; Zhang, S.; Liu, T. Intraguild predation on the aphid parasitoid Aphelinus asychis by the ladybird Harmonia axyridis. BioControl 2017, 62, 61–70.
- Khan, J.; Khan, A.; Ahmed, N.; Alhag, S.K.; Almadiy, A.A.; Sayed, S.; Alam, P.; Ullah, F. Age and stage-specific life table parameters of Harmonia dimidiata (Coleoptera: Coccinellidae) fed on Rhopalosiphum padi (Hemiptera: Aphididae) at different temperatures. Egypt. J. Biol. Pest Control 2022, 32, 113.
- Ahmed, N.; Chamila-Darshanee, H.L.; Fu, W.Y.; Hu, X.S.; Fan, Y.; Liu, T.X. Resistance of seven cabbage cultivars to green peach aphid (Hemiptera: Aphididae). J. Econ. Entomol. 2018, 111, 909–916.
- Khater, H.F. Prospects of botanical biopesticides in insect pest management. Pharmacologia 2012, 3, 641–656.
- Ullah, M.; Ullah, F.; Khan, M.A.; Ahmad, S.; Jamil, M.; Sardar, S.; Tariq, K.; Ahmed, N. Efficacy of various natural plant extracts and the synthetic insecticide cypermethrin 25EC against Leucinodes orbonalis and their impact on natural enemies in brinjal crop. Int. J. Trop. Insect Sci. 2022, 42, 173–182.
- Iqbal, T.; Ahmed, N.; Shahjeer, K.; Ahmed, S.; Al-Mutairi, K.A.; Khater, H.F.; Ali, R.F. Botanical Insecticides and their Potential as Anti-Insect/Pests: Are they Successful against Insects and Pests? In Global Decline of Insects; IntechOpen: London, UK, 2021.
- Ahmed, N.; Alam, M.; Saeed, M.; Ullah, H.; Iqbal, T.; Al-Mutairi, K.A.; Shahjeer, K.; Ullah, R.; Ahmed, S.; Ahmed, N.A.H.; et al. Botanical Insecticides Are a Non-Toxic Alternative to Conventional Pesticides in the Control of Insects and Pests, In Global Decline of Insects; El-Shafie, H., Ed.; IntechOpen: London, UK, 2021.
- Jan, Q.; Khan, I.A.; Al-Shuraym, L.A.; Alshehri, M.A.; Ahmed, N.; Saeed, M.; El-Sharnouby, M.; Sayed, S. Comparative conventional preventive strategies for insect pest of okra. Saudi J. Biol. Sci. 2022, 29, 3114–3121.
- Khater, H.F. Ecosmart Biorational Insecticides: Alternative Insect Control Strategies. In Insecticides-Advances in Integrated Pest Management; Preveen, F., Ed.; InTech: Rijeka, Croatia, 2012; pp. 17–60. ISBN 978-953-307-780.
- Khater, H.F. Bioactivity of Essential Oils as Green Biopesticides: Recent Global Scenario. Recent Prog. Med. Plants 2013, 37, 151–218.
- Darshanee, H.L.; Ren, H.; Ahmed, N.; Zhang, Z.F.; Liu, Y.H.; Liu, T.X. Volatile-mediated attraction of greenhouse whitefly Trialeurodes vaporariorum to tomato and eggplant. Front. Plant Sci. 2017, 8, 1285.
- Ahmed, N.; Darshanee, H.L.; Khan, I.A.; Zhang, Z.F.; Liu, T.X. Host selection behavior of the green peach aphid, Myzus persicae, in response to volatile organic compounds and nitrogen contents of cabbage cultivars. Front. Plant Sci. 2019, 10, 79.
- Saeed, M.; Ahmad, T.; Alam, M.; Al-Shuraym, L.A.; Ahmed, N.; Alshehri, M.A.; Ullah, H.; Sayed, S.M. Preference and performance of peach fruit fly (Bactrocera Zonata) and Melon fruit fly (Bactrocera Cucurbitae) under laboratory conditions. Saudi J. Biol. Sci. 2022, 29, 2402–2408.
- Gao, X.W.; Wu, M.X.; Sun, J.H.; Wang, Y.C. Empedobacter brevis on insecticidal effect and technology for Cnaphalocrocis medinalis Guenee. Pest Sci. Admin. 2012, 33, 43–50.
- Zhai, H.W.; Zhao, Y.F.; Gao, X.W.; Wu, M.X. Efficacy of Empedobacter brevis in controlling rice stem borer. Biol. Disast. Sci. 2013, 36, 291–294.
- Liu, Q.; Xu, J.; Wang, Y.; Li, C.; Han, G.; Qi, J.; Sun, J.; Ma, T. Synergism of CmGV and Bacillus thuringiensis against larvae of Cnaphalocrocis medinalis Güenée. J. Yangzhou Univ. Agric. Life Sci. Ed. 2013, 34, 89–93.
- Zhai, H.; Chai, Y. Effect of Mamestra brassicae nuclear polyhedrosis virus suspending agent on Chilo suppressalis in Rice. North Rice 2013, 43, 64–65.
- Mo, J.; Ding, W.B.; Li, Y.Z.; Li, G.H. Control effect of 9% 12 α-hydroxy-rotenone EW against three pests of Rice. Hunan Agric Sci. 2014, 10, 40–42.
- Wang, G.R.; Han, R.P.; Shen, Q.; Yang, W.T.; Wang, G.D.; Lu, Z.X.; Zheng, X.S. Effects of modified fertilizer application on rice pests, diseases and yields. China Rice 2015, 21, 94–97.
- Yuan, X.H.; Song, L.W.; Zhang, J.J.; Zang, L.S.; Zhu, L.; Ruan, C.C.; Sun, G.Z. Performance of four Chinese Trichogramma species as biocontrol agents of the rice striped stem borer, Chilo suppressalis, under various temperature and humidity regimes. J. Pest Sci. 2012, 85, 497–504.
- Zhang, S.; Jia, X.W.; Sun, S.F.; Pang, Y.; Chen, Q.J.; Yang, K. Phylogenetic analysis and epidemiologic investigation of a Cnaphalocrocis medinalis granulovirus strain. J. Environ. Entomol. 2014, 36, 756–762.
- Li, D.S.; Yuan, X.; Zhang, B.X.; Zhao, Y.; Song, Z.W.; Zuo, C. Report of using unmanned aerial vehicle to release Trichogramma. Chin. J. Biol. Control 2013, 29, 455–458.
- Tian, J.C.; Wang, G.R.; Zheng, X.S.; Wang, G.W.; Lu, Z.X.; Xu, H.X.; Yang, Y.J.; Lu, Y.H. A Trichogramma Release Device with a Sugar Feeding Device. Chinese Patent ZL201420667926.1, 2015.
- Du, Y.J.; Guo, R.; Han, Q.R. The application techniques of insect sex pheromone control of rice stem borer and rice leaf folder. China Plant Prot. 2013, 33, 39–42.
- Zhu, P.Y.; Sheng, X.Q.; Feng, F.; Li, Y.H.; Chen, G.H. Trapping technique of insect sex pheromone on striped stem borer and rice leaf folder. J. Zhejiang Agric. Sci. 2013, 54, 825–826.
- Yang, F.A.; Sheng, C.F.; Wei, Y.B. Study on mating disruption with synthetic sex pheromone for controling rice stem borer. Plant Prot. 2001, 27, 4–6.
- Xia, Y.Z.; Sun, W.Y. Trapping and killing effects of Vetiveria zizanioides on rice stem borers. Zhejiang Agric. Sci. 2012, 1, 1693–1695.
- Liang, Q.; Lu, Y.H.; He, X.C.; Zheng, X.S.; Xu, H.X.; Yang, Y.J.; Tian, J.C.; Lu, Z.X. Mini review of the significance of trap crop in insect pest management. J. Biosaf. 2015, 24, 184–193.
- Yang, Z.P.; Liu, X.Y.; Huang, H.; Liu, D.Z.; Hu, L.D.; Su, W.; Tan, S.Q. A study on the influence of rice-duck intergrowth on spider, rice diseases, insect and weeds in rice-duck complex ecosystems. ACTA Ecol. Sin. 2004, 24, 2756–2760.
- Long, P.; Huang, H.; Liao, X.; Fu, Z.; Zheng, H.; Chen, A.; Chen, C. Mechanism and capacities of reducing ecological cost through Rice–duck cultivation. J. Sci. Food Agric. 2013, 93, 2881–2891.
- Zhu, P.; Zheng, X.; Yao, X.; Xu, H.; Zhang, F.; Chen, G.; Lü, Z. Ecological engineering technology for enhancement of biological control capacity of egg parasitoids against rice plant hoppers in paddy fields. China Plant Prot. 2015, 7, 27–32.
- Tao, L.Y.; Yu, X.P.; Wu, G.R. Preliminary monitoring the biotypes of the brown planthopper Nilaparvata lugens Stål in China. Sci. Agric. Sin. 1992, 25, 9–13.
- Liu, X.Y.; Liu, D.Z.; Chen, Y.F.; Huang, H.; Zhong, L.; Yu, J.B. The character of rice roots in rice-duck-fish commensalisms ecosystem and its economic benefit. J. Hunan Agric. Univ. Nat. Sci. 2005, 31, 314–316.
This entry is offline, you can click here to edit this entry!
