Retromode is a relatively new retinal-imaging technique that is based on the transillumination principle and is obtained with a scanning laser ophthalmoscope that uses light in the infrared spectrum. The laser light penetrates into the deep retinal layers and the choroid. Retromode images are captured with a laterally displaced aperture, and the detector captures only the scattered light. The result is a high-contrast pseudo-three-dimensional image. Age-related macular degeneration (AMD) is a disabling retinal disease. AMD is characterized in its early stage by small and intermediate drusen formation, while the signs of intermediate AMD are large drusen and/or pigmentary abnormalities. Late AMD has two forms, geographic atrophy, which is the advanced form of dry AMD, and wet AMD. Most of the lesions of AMD are located in the outer layers of the retina. This new imaging method can provide a glimpse of the deep retinal layers’ topographic changes in a non-invasive, fast, and effective way that can match the other imaging tools available.
1. Introduction
Age-related macular degeneration (AMD) is a retinal disease that causes central vision loss [
1]. It is a public health issue because of the large number of affected patients, with 1.8 million cases in 2020. Following glaucoma, it is the second leading cause of irreversible blindness in adults over 50 years old [
2]. AMD is a multifactorial disorder. The risk factors are age, female sex, white race, smoking, and low antioxidant intake [
1]. Genetic factors involving the complement pathway and the age-related maculopathy susceptibility 2 locus (ARMS2) genes are usually present in most of the patients with late-stage disease [
3].
Ferris et al. stated that normal aging changes in the retina consist of drupelets (small drusen), and these do not present an increased risk of developing the advanced retinal disease. According to the Beckman classification, early AMD is represented by medium drusen (>63 microns and <125 microns), while large drusen (>125 microns) and pigmentary abnormalities (hypo- or hyperpigmentation) are considered intermediate AMD. Late-stage disease is represented by its two variants of evolution: exudative (neovascular or wet) AMD or geographic atrophy (GA) [
4].
The diagnosis of AMD was classically made based on clinical examination findings and relied on color fundus photography (CFP). Fluorescein angiography (FA) used to be the main investigation for treatment decisions [
5]. Now the gold standard tool used by retinal specialists in the diagnosis and monitoring of the disease is spectral domain optical coherence tomography (SD-OCT), a non-invasive investigation that offers high-resolution cross-sectional images of the retina [
6], which are used for detecting various biomarkers and classifications of AMD [
7]. FA is still useful because it can confirm neovascularization, and it can also detect leakage, while newer methods such as optical coherence tomography angiography can not [
8].
In the era of multimodality, fundus autofluorescence (FAF) is another non-invasive tool that proved to be very useful in detecting early and late AMD changes by assessing the lipofuscin quantity in the retinal pigmentary epithelium (RPE) [
9,
10]. Since technology is developing at a fast rate and there are newer methods to investigate AMD patients, it is worth turning a spotlight on retromode, another non-invasive imaging method of the retina.
2. Retromode Imaging
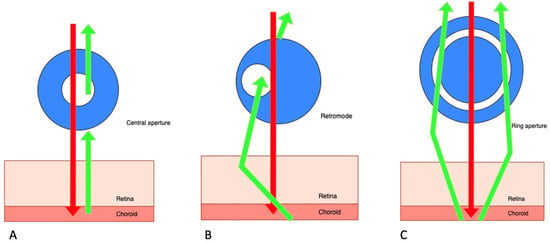
Retromode is a relatively new imaging modality that uses the principle of transillumination [
11]. The retromode images are captured with a scanning laser ophthalmoscope (SLO), which uses a laser light with a deviated aperture instead of a central aperture (see
Figure 1) [
11]. The aperture can be deviated laterally to the left (DL), to the right (DR), or annular (RA—using a ring aperture). The scattered light comes from the choroid and deep retina, and it does not return directly to the detector, so the result is a pseudo-three-dimensional (pseudo3D) image [
11].
Figure 1. Scanning laser ophthalmoscope: three different aperture types: (A) confocal (with a central aperture). (B) retromode (with a laterally deviated aperture to the left or to the right), and, respectively, (C) the ring aperture.
Images captured with a confocal SLO through a central aperture have good contrast and resolution. Both the reduced-size aperture, which is central in relation to the detector, and the scanning laser beam are focused on the same tissue location [
11].
The latest system that can produce this kind of image is the Mirante SLO/OCT (Nidek Co., Gamagori, Japan). Retromode imaging was also available on an earlier version of this platform, the F-10 SLO (Nidek Co., Gamagori, Japan) system. The Mirante platform uses infrared laser illumination (790 nm) in order to capture the retromode images. The infrared laser light has the ability to penetrate deeper into the tissues than visible light and gets to the level of the outer retina layers, the RPE, and the choroid [
11,
12,
13,
14,
15]. The lesions of the deep retinal and choroid tissues are highlighted with the shades of the laterally displaced light. The result is a high-contrast image obtained with DR and DL modes, which have the advantage of identifying more subtle abnormalities in the outer retina, the RPE, and the choroid. The images produced with the annular ring aperture are not as clear, and the contrast between structures is reduced [
11].
The pseudo3D effect is in fact a virtual illusion that is induced by the shades and borders on one side of the structures illustrated in the images, which gives the observer a sense of the depth of the lesions [
11,
15].
3. Age-Related Macular Degeneration Imaged with Retromode
Age-related macular degeneration is represented by heterogenous and pathognomonic manifestations, depending on the stage of the disease of the patient. Each of the clinical entities found in AMD will be described, along with the utility of this method in the diagnosis and monitoring of the patients found in literature, and illustrative images for the different clinical entities.
3.1. Early and Intermediate AMD
Early and intermediate AMD patients usually do not present any symptoms. Some of them may report mild image distortion when performing nearby tasks such as reading [
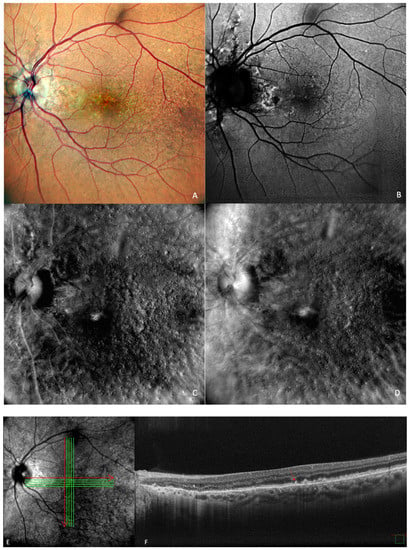
1]. In early AMD (
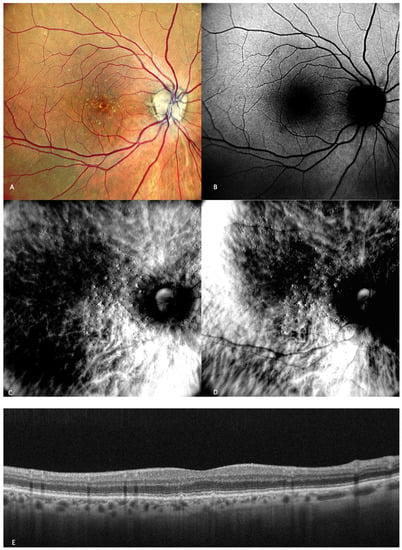
Figure 2), the main finding is medium-sized drusen. In patients with intermediate AMD (
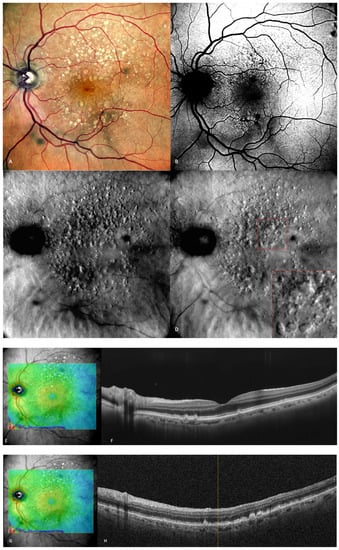
Figure 3), medium and large drusen appear, and also pigmentary abnormalities can be detected.
Figure 2. Early AMD. Multimodal imaging of small and medium drusen. (A) Color scanning laser ophthalmoscope photography shows multiple small drusenoid round, yellow deposits. (B) On the fundus autofluorescence, drusen hardly cause any effect. (C) Retromode deviated to right and (D) retromode deviated to left display drusen as small elevations or depressions, respectively. Drusen number and extension are obviously more easily visualized on the retromode images than on the cSLO image. (E) SD-OCT section, which shows drusen as small elevations of the RPE.
Figure 3. Intermediate AMD. (
A) Multiple medium and large drusen can be seen on the cSLO image. (
B) On green FAF, large drusen appear as hyper-autofluorescent patches. (
C) Retromode deviated right depicting elevated, large drusen. (
D) Retromode deviated left shows a “moon surface” appearance (zoomed image in the red square). (
E,
G) En-face OCT showing the location of the section in (
F,
H) SD-OCT showing multiple small, medium, and large drusen.
3.1.1. Drusen
Drusen are incipient changes in the fundus that are indicative of age-related macular degeneration, and early identification is anticipated to become more crucial given potential therapies in the future.
Clinically, drusen show up as yellow-white deposits under the retina during biomicroscopy. The gold standard for determining the clinical stage of non-neovascular AMD has been color fundus photography (CFP) for a long time [
17,
18,
19].
Drusen are identified by the buildup of extracellular material between the basal lamina of the RPE and the inner collagenous layer of Bruch’s membrane. Drusen can be classified depending on their consistency, size, and margin into four categories: hard drusen, soft drusen, cuticular drusen, and mineralized (calcified) drusen. Subretinal drusenoid deposits (also known as reticular pseudodrusen) are another entity, and they are localised above the RPE [
18]. Large drusen are well-known to be a risk factor for developing advanced AMD [
20,
21,
22], while hard drusen are not incriminated to be one.
3.1.2. Subretinal Drusenoid Deposits
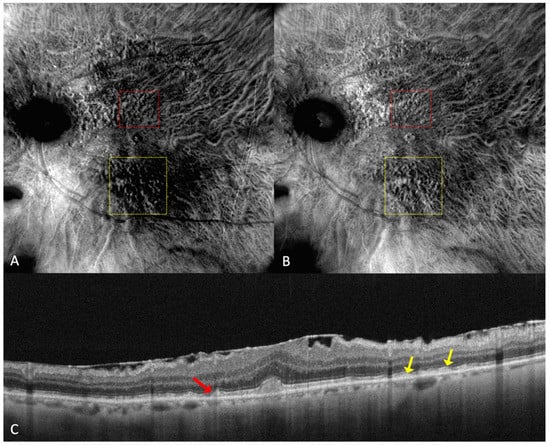
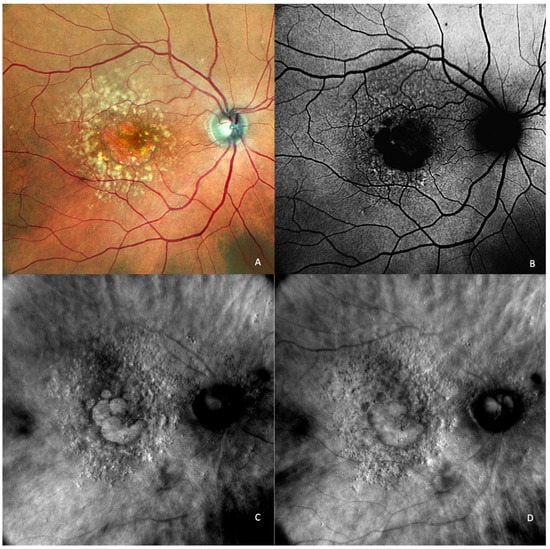
Reticular pseudodrusen or subretinal drusenoid deposits (SDDs—see
Figure 4 and
Figure 5) are located above the RPE and are considered to be a high-risk factor for the progression of AMD to the late forms: GA or neovascularization [
26,
27]. SDDs have an inclination to appear superior to the fovea and spare the more central macula [
28]. SDDs were classified by Suzuki et al. in three main subtypes: dot, ribbon, and midperipheral [
29]. These entities are best detected with SD-OCT and with infrared reflectance, and it is recommended to use multiple imaging modalities in order to diagnose SDDs [
30].
Figure 4. (
A) On the retromode image DR dot SDDs are round, hyporeflective lesions, and ribbon SDDs are difficult to see. (
B) Retromode image DL shows each dot SDD as “hyporeflective core” with a “hyperreflective halo” [
24], while the ribbon SDDs tend to have a reticular structure. (
C) SD-OCT shows dot SDD (red arrow), ribbon SDDs (yellow arrows), and also a hyper-reflective layer on the retinal surface.
Figure 5. Drusen and dot subretinal drusenoid deposits (SDD) in a patient with co-existent angioid streaks: (
A) cSLO showing multiple drusen and SDDs, with peripapillary radiating irregular lines. (
B) In green FAF, temporal to the fovea, drusen are seen as hypo- and hyper-autofluorescent round lesions. (
C) In retromode deviated right, elevated lesions can be seen, and in (
D) retromode deviated left, small, depressed lesions are detected. (
E) En face OCT of (
F) SD-OCT shows multiple small, medium, large drusen and also dot SDD (red arrow).
3.1.3. Retinal Pigment Epithelium Abnormalities
Another significant characteristic of non-neovascular AMD is represented by RPE abnormalities, which indicate a higher likelihood of development into atrophy and NV [
20,
21,
22].
Bruch’s membrane thickens and loses permeability as a result of drusen deposition in tandem with other structural and biochemical alterations related to AMD pathogenesis, such as chronic activation of the complement cascade and inflammation [
32].This causes the choroidal vasculature to thin and obstructs waste exchange to the choroid as well as nutrient transport to the retina. In early or intermediate illness, these actions, along with late neurodegenerative changes in the photoreceptor-RPE complex, cause pigmentary abnormalities of the RPE, including hypo- or hyperpigmentation [
33]. In the normal aging eye, RPE cell counts decline, though this loss is much less than that experienced by individuals with AMD [
34]. The RPE’s pigmentation and shape change as a result of an increase in lipofuscin granules and a decline in melanosomes [
34].
3.2. Late AMD
Patients with late AMD present with very reduced central visual acuity. End-stage AMD can be divided into two categories based on whether choroidal neovascularization is present or absent: non-neovascular (known as non-exudative, atrophic, or dry) or neovascular AMD (also referred to as exudative or wet) [
1].
3.2.1. Geographic Atrophy
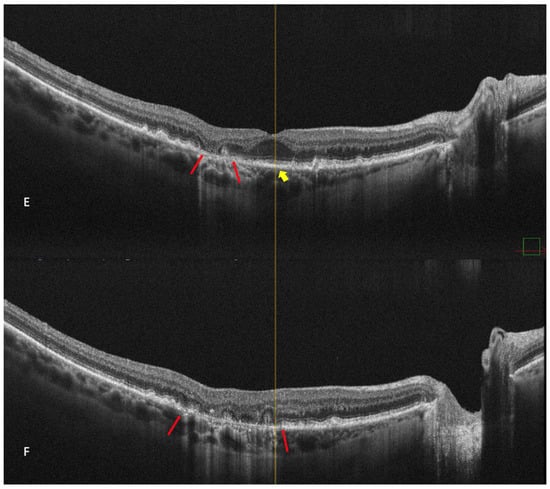
In 1970, Gass first used the phrase “geographic zones of atrophy” to refer to “senile macular choroidal degeneration” [
39]. Geographic atrophy (GA) is the progressive end-stage form of dry AMD and is represented by a round area where RPE pigment is reduced or absent and where the choroidal vessels are readily apparent (
Figure 6). The smallest area that geographic atrophy can affect is a circle with a diameter of roughly 175 microns [
40]. A notable decrease in visual acuity over time in many eyes is linked to geographic atrophy [
41]. Even when they were already substantial at baseline, areas of atrophy continue to grow over time [
41]. GA has a mean rate of progression of around 1.95 mm
2/year [
42]. Color fundus photography and fundus autofluorescence are two of the main tools used for the diagnosis and monitoring of geographic atrophy [
43].
Figure 6. Geographic atrophy. (
A) cSLO image showing round patches of GA where the choroidal vessels are visible, surrounded by large and medium drusen. (
B) Green FAF showing confluent hypo-autofluorescent areas. (
C) DR and (
D) DL retromode images of GA seen as round patches with homogenous reflectivity where the underlying choroidal vessels are visible. (
E) SD-OCT showing RPE and outer retinal atrophy temporal to the fovea (red arrows) with foveal sparing (yellow short arrow). (
F) SD-OCT showing large area of RPE and outer retinal atrophy (red arrows).
3.2.2. Exudative AMD
Wet AMD is represented by neovascularization. It includes several common lesions, such as the presence of intraretinal, subretinal, sub-RPE fluid, RPE detachment, hard exudates, retinal haemorrhages, or disciform fibrotic scars [
1].
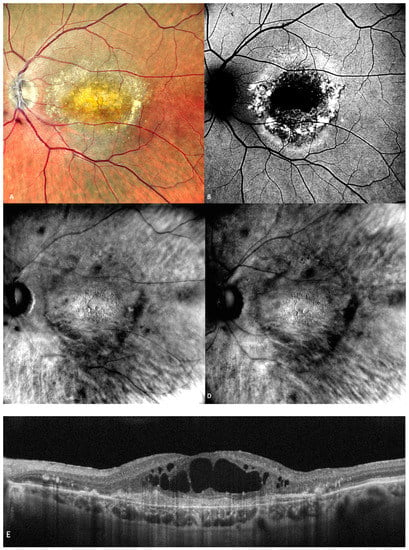
Retromode illumination imaging modality’s ability to detect changes in the retina that appear in wet AMD was evaluated by Pilotto et al. [
45]. Neuroretinal detachment (NRD) can be seen on the retromode image as an elevated area (62.5% sensibility; 66.7% specificity) [
45]. Pigment epithelium detachment (PED) can be detected with retromode illumination as an elevated area (100% sensibility; 69% specificity) [
45]. Cystoid macular edema (CME—See
Figure 7) is another entity well-detected with retromode illumination (87.5% sensibility; 100% specificity) [
45]. Epiretinal membrane was well-detected with retromode (66.7% sensibility; 100% specificity) [
45].
Figure 7. (A) The cSLO shows subfoveal fibrosis surrounded by exudates. (B) Mottled, irregular autofluorescence pattern with a central hypo-autofluorescent core. (C) Retromode DR and (D) Retromode DL images showing large, oval, concave, and, respectively, convex cystoid spaces. (E) Large intraretinal cystoid spaces seen over an area of subretinal fibrosis on SD-OCT.
This entry is adapted from the peer-reviewed paper 10.3390/medicina59040647