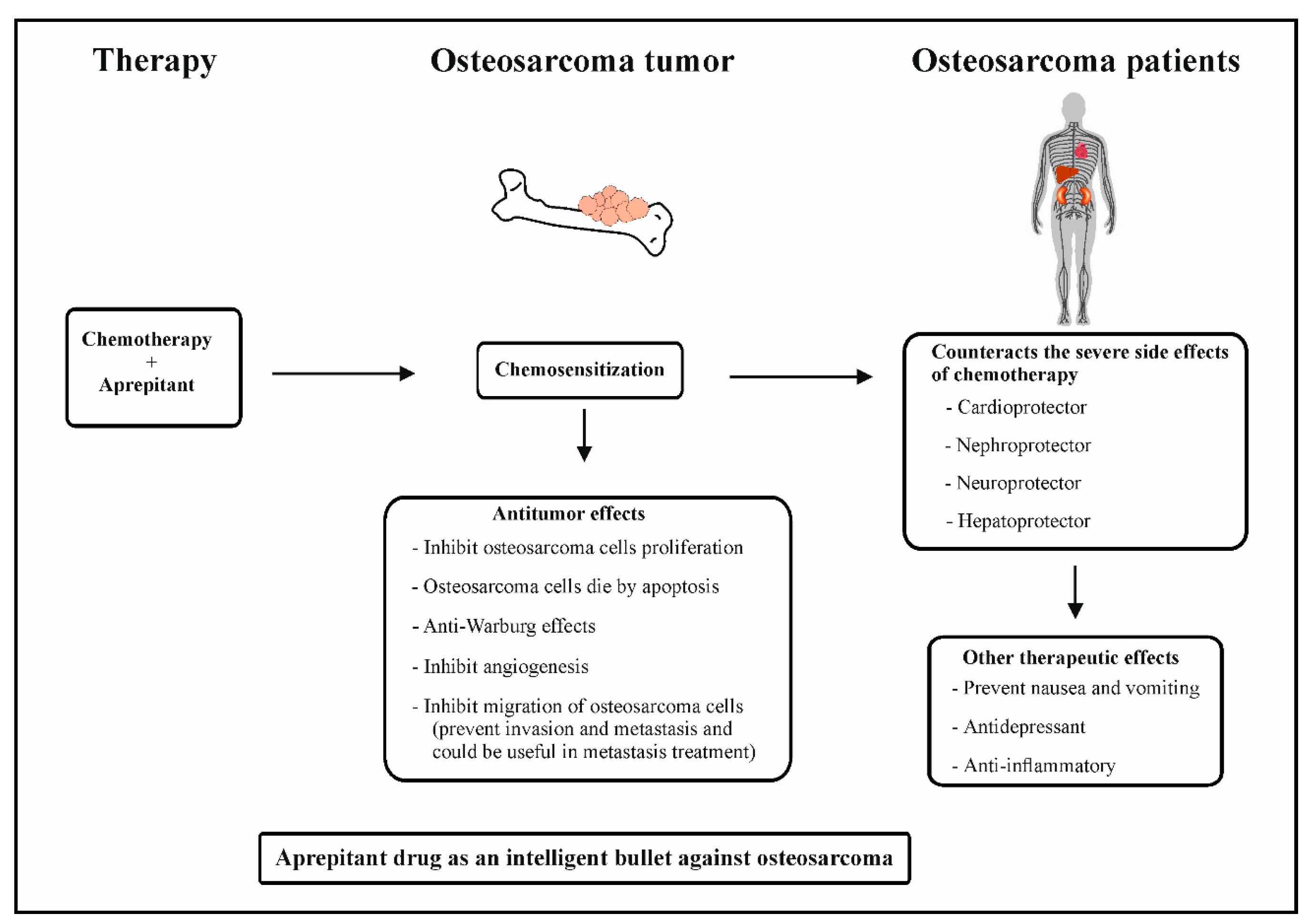
Osteosarcoma is a bone tumor predominantly affecting children and adolescents with high malignant potential. It is a cause of serious public health challenges due to its high morbidity rates and metastatic potential. Metastasis in osteosarcoma may manifest either during treatment of the primary tumor, shortly after treatment, or a long time after the end of the treatment. So far, there are no therapeutics that can prevent or treat osteosarcoma metastasis. The peptide substance P (SP) and its high-affinity receptor, Neurokinin-1 (NK-1R), are known to positively correlate with osteosarcoma progression. Osteosarcoma cells overexpress NK-1R. SP is known to elicit the proliferation of osteosarcoma cells and induce angiogenesis and migration, leading to the invasion and metastasis of tumor cells. In contrast, NK-1R antagonists, such as aprepitant, inhibit the proliferation and induce the apoptosis of osteosarcoma cells. Aprepitant is also known to inhibit the migration of osteosarcoma cells, as well as reduce the expression levels and activities of transcriptional regulators of metastasis-related genes such as matrix metalloproteinases (MMP-2 and MMP-9), vascular endothelial growth factor (VEGF), and nuclear factor kappa B (NF-κB). These preceding studies highlighted the antimetastatic role of aprepitant in osteosarcoma Moreover, combination therapy consisting of chemotherapy and NK-1R antagonist increases the chemosensitization of osteosarcoma cells.
- osteosarcoma
- NK-1 receptor
- apoptosis
1. Role of Neurokinin-1 Antagonists as Antiproliferative and Proapoptotic Agents in Osteosarcoma
2. Role of Neurokinin-1 Antagonists as Antiangiogenic Agents in Osteosarcoma
3. Role of Neurokinin-1 Antagonists as Agents That Counteract the Warburg Effect in Osteosarcoma
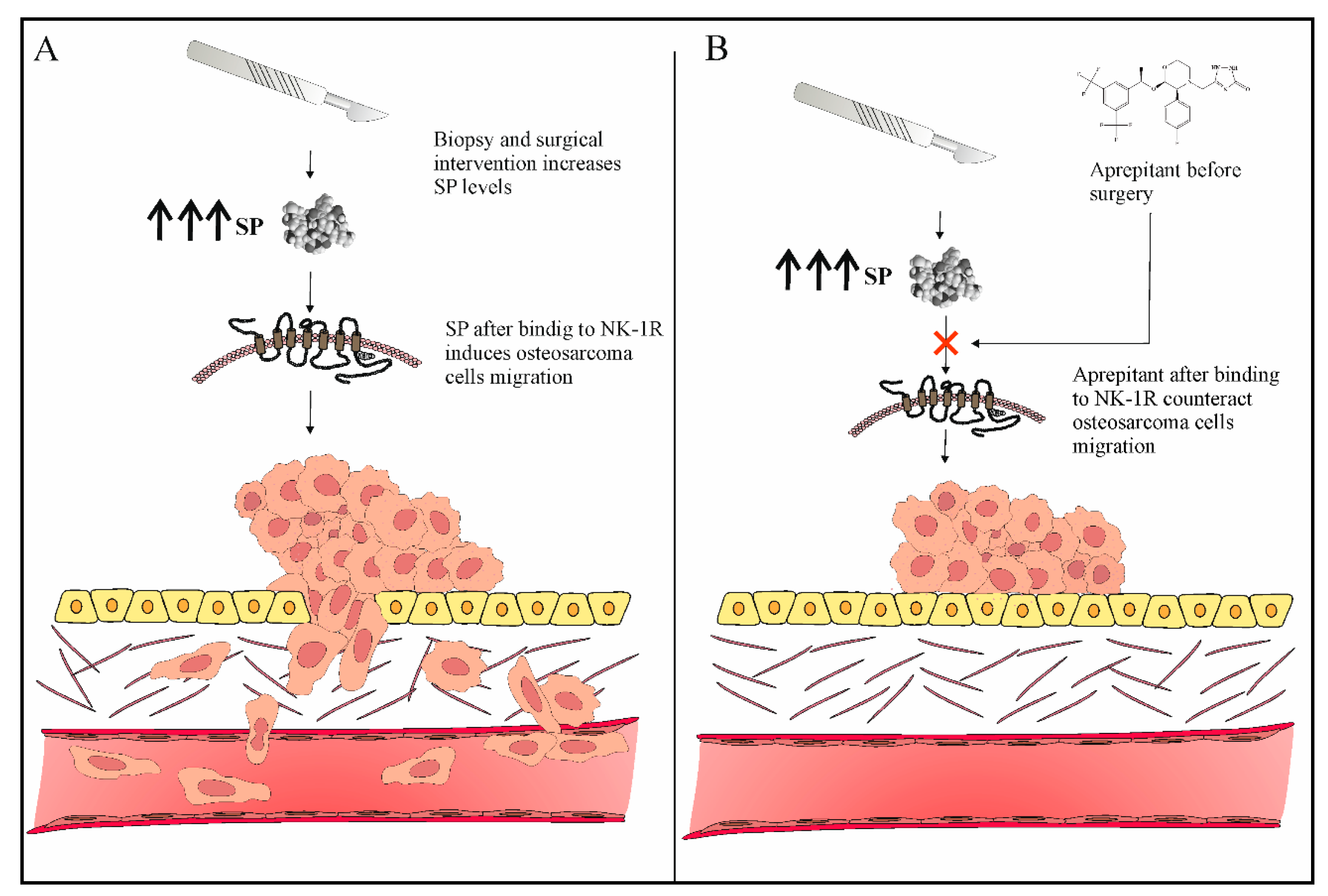
4. Role of Neurokinin-1 Antagonists as Antimetastatic Agents in Osteosarcoma
5. Neurokinin-1 Antagonists as Intelligent Drugs in Osteosarcoma Therapy
This entry is adapted from the peer-reviewed paper 10.3390/jcm12062135
References
- Muñoz, M.; Berger, M.; Rosso, M.; Gonzalez-Ortega, A.; Carranza, A.; Coveñas, R. Antitumor activity of neurokinin-1 receptor antagonists in MG-63 human osteosarcoma xenografts. Int. J. Oncol. 2013, 44, 137–146.
- Alsaeed, M.A.; Ebrahimi, S.; Alalikhan, A.; Hashemi, S.F.; Hashemy, S.I. The Potential In Vitro Inhibitory Effects of Neurokinin-1 Receptor (NK-1R) Antagonist, Aprepitant, in Osteosarcoma Cell Migration and Metastasis. BioMed Res. Int. 2022, 2022, 8082608.
- Zeng, M.; Zhou, J.; Wen, L.; Zhu, Y.; Luo, Y.; Wang, W. The relationship between the expression of Ki-67 and the prognosis of osteosarcoma. BMC Cancer 2021, 21, 210.
- Berger, M.; Neth, O.; Ilmer, M.; Garnier, A.; Salinas-Martín, M.V.; de Agustin Asencio, J.C.; von Schweinitz, D.; Kappler, R.; Muñoz, M. Hepatoblastoma cells express truncated neurokinin-1 receptor and can be growth inhibited by aprepitant in vitro and in vivo. J. Hepatol. 2014, 60, 985–994.
- Hennig, I.M.; Laissue, J.A.; Horisberger, U.; Reubi, J.-C. Substance-P receptors in human primary neoplasms: Tumoral and vascular localization. Int. J. Cancer 1995, 61, 786–792.
- Ziche, M.; Morbidelli, L.; Pacini, M.; Geppetti, P.; Alessandri, G.; Maggi, C.A. Substance P stimulates neovascularization in vivo and proliferation of cultured endothelial cells. Microvasc. Res. 1990, 40, 264–278.
- Warburg, O. On the Origin of Cancer Cells. Science 1956, 123, 309–314.
- Fan, J.; Mei, J.; Zhang, M.-Z.; Yuan, F.; Li, S.-Z.; Yu, G.-R.; Chen, L.-H.; Tang, Q.; Xian, C.J. Clinicopathological significance of glucose transporter protein-1 overexpression in human osteosarcoma. Oncol. Lett. 2017, 14, 2439–2445.
- Fan, J.; Zhou, J.-Q.; Yu, G.-R.; Lu, D.-D. Glucose Transporter Protein 1–Targeted RNA Interference Inhibits Growth and Invasion of the Osteosarcoma Cell Line MG63 In Vitro. Cancer Biother. Radiopharm. 2010, 25, 521–527.
- Jian, F.; Yuan, F.; Jiong, M.; Zhu, X.-Z.; Yu, G.-R.; Lu, D.-D. Silencing of Glucose Transporter Protein-1 by RNA Interference Inhibits Human Osteosarcoma Mg63 Cells Growth in vivo. Technol. Cancer Res. Treat. 2014, 14, 243–248.
- Maschek, G.; Savaraj, N.; Priebe, W.; Braunschweiger, P.; Hamilton, K.; Tidmarsh, G.F.; De Young, L.R.; Lampidis, T.J. 2-Deoxy-d-glucose Increases the Efficacy of Adriamycin and Paclitaxel in Human Osteosarcoma and Non-Small Cell Lung Cancers In Vivo. Cancer Res. 2004, 64, 31–34.
- Muñoz, M.; Coveñas, R. Involvement of substance P and the NK-1 receptor in cancer progression. Peptides 2013, 48, 1–9.
- Medrano, S.; Gruenstein, E.; Dimlich, R.V. Substance P receptors on human astrocytoma cells are linked to glycogen breakdown. Neurosci. Lett. 1994, 167, 14–18.
- Odri, G.A.; Tchicaya-Bouanga, J.; Yoon, D.J.Y.; Modrowski, D. Metastatic Progression of Osteosarcomas: A Review of Current Knowledge of Environmental versus Oncogenic Drivers. Cancers 2022, 14, 360.
- Xie, L.; Ji, T.; Guo, W. Anti-angiogenesis target therapy for advanced osteosarcoma (Review). Oncol. Rep. 2017, 38, 625–636.
- Kumta, S.M.; Huang, L.; Cheng, Y.Y.; Chow, L.T.; Lee, K.M.; Zheng, M.H. Expression of VEGF and MMP-9 in giant cell tumor of bone and other osteolytic lesions. Life Sci. 2003, 73, 1427–1436.
- Tohme, S.; Simmons, R.L.; Tsung, A. Surgery for cancer: A trigger for metastases. Cancer Res. 2017, 77, 1548–1552.
- Hiller, J.G.; Perry, N.J.; Poulogiannis, G.; Riedel, B.; Sloan, E.K. Perioperative events influence cancer recurrence risk after surgery. Nat. Rev. Clin. Oncol. 2018, 15, 205–218.
- Loughran, C.F.; Keeling, C.R. Seeding of tumour cells following breast biopsy: A literature review. Br. J. Radiol. 2011, 84, 869–874.
- Gharaee, N.; Pourali, L.; Jafarian, A.H.; Hashemy, S.I. Evaluation of serum level of substance P and tissue distribution of NK-1 receptor in endometrial cancer. Mol. Biol. Rep. 2018, 45, 2257–2262.
- Muñoz, M.; Coveñas, R. The Neurokinin-1 Receptor Antagonist Aprepitant: An Intelligent Bullet against Cancer? Cancers 2020, 12, 2682.
- Muñoz, M.; Rosso, M. The NK-1 receptor antagonist aprepitant as a broad spectrum antitumor drug. Investig. New Drugs 2010, 28, 187–193.
- Giagnuolo, G.; Buffardi, S.; Rossi, F.; Petruzziello, F.; Tortora, C.; Buffardi, I.; Marra, N.; Beneduce, G.; Menna, G.; Parasole, R. Single center experience on efficacy and safety of Aprepitant for preventing chemotherapy-induced nausea and vomiting (CINV) in pediatric Hodgkin Lymphoma. PLoS ONE 2019, 14, e0215295.
- Kose, D.; Un, H.; Ugan, R.A.; Halici, Z.; Cadirci, E.; Tastan, T.B.; Kahramanlar, A. Aprepitant: An antiemetic drug, contributes to the prevention of acute lung injury with its anti-inflammatory and antioxidant properties. J. Pharm. Pharmacol. 2021, 73, 1302–1309.
- Tebas, P.; Tuluc, F.; Barrett, J.S.; Wagner, W.; Kim, D.; Zhao, H.; Gonin, R.; Korelitz, J.; Douglas, S.D. A Randomized, Placebo Controlled, Double Masked Phase IB Study Evaluating the Safety and Antiviral Activity of Aprepitant, a Neurokinin-1 Receptor Antagonist in HIV-1 Infected Adults. PLoS ONE 2011, 6, e24180.
- Spitsin, S.; Tebas, P.; Barrett, J.S.; Pappa, V.; Kim, D.; Taylor, D.; Evans, D.L.; Douglas, S.D. Antiinflammatory effects of aprepitant coadministration with cART regimen containing ritonavir in HIV-infected adults. JCI Insight 2017, 2, e95893.
- Kramer, M.S.; Cutler, N.; Feighner, J.; Shrivastava, R.; Carman, J.; Sramek, J.J.; Reines, S.A.; Liu, G.; Snavely, D.; Wyatt-Knowles, E.; et al. Distinct Mechanism for Antidepressant Activity by Blockade of Central Substance P Receptors. Science 1998, 281, 1640–1645.
- Robinson, P.; Coveñas, R.; Muñoz, M. Combination Therapy of Chemotherapy or Radiotherapy and the Neurokinin-1 Receptor Antagonist Aprepitant: A New Antitumor Strategy? Curr. Med. Chem. 2023, 30, 1798–1812.
