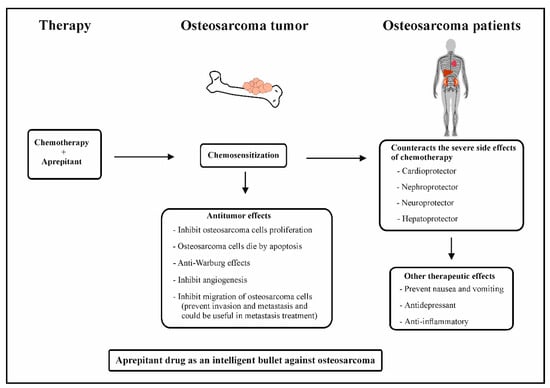
Osteosarcoma is a bone tumor predominantly affecting children and adolescents with high malignant potential. It is a cause of serious public health challenges due to its high morbidity rates and metastatic potential. Metastasis in osteosarcoma may manifest either during treatment of the primary tumor, shortly after treatment, or a long time after the end of the treatment. So far, there are no therapeutics that can prevent or treat osteosarcoma metastasis. The peptide substance P (SP) and its high-affinity receptor, Neurokinin-1 (NK-1R), are known to positively correlate with osteosarcoma progression. Osteosarcoma cells overexpress NK-1R. SP is known to elicit the proliferation of osteosarcoma cells and induce angiogenesis and migration, leading to the invasion and metastasis of tumor cells. In contrast, NK-1R antagonists, such as aprepitant, inhibit the proliferation and induce the apoptosis of osteosarcoma cells. Aprepitant is also known to inhibit the migration of osteosarcoma cells, as well as reduce the expression levels and activities of transcriptional regulators of metastasis-related genes such as matrix metalloproteinases (MMP-2 and MMP-9), vascular endothelial growth factor (VEGF), and nuclear factor kappa B (NF-κB). These preceding studies highlighted the antimetastatic role of aprepitant in osteosarcoma Moreover, combination therapy consisting of chemotherapy and NK-1R antagonist increases the chemosensitization of osteosarcoma cells.
1. Role of NK-1R Antagonists as Antiproliferative and Proapoptotic Agents in Osteosarcoma
There are three NK-1R antagonists that have shown activity in the treatment of osteosarcoma in vitro and in vivo: L-733,060, L-732,138, and FDA-approved aprepitant for the treatment of chemotherapy-induced nausea and vomiting (CINV) [
5]. The above NK-1R antagonists inhibit proliferation and induce apoptosis of osteosarcoma cells in a concentration-dependent manner [
5,
13] (
Figure 1). Importantly L-733,060 was shown to decrease tumor volume in a murine preclinical model of osteosarcoma [
5]. Positive Ki-67 expression was demonstrated to be associated with stage, distant metastasis, and overall survival of osteosarcoma, thus implicating its potential as a biomarker to predict prognosis and guide clinical therapy for osteosarcoma [
23]. Similarly, in a hepatoblastoma xenograft mouse model, treatment with NK-1R antagonist aprepitant at 80 mg/kg/day for 24 days led to a significant reduction in tumor volume and weight, as well as in Ki-67-positive cells [
8]. These studies highlight the potential of the NK-1R antagonist aprepitant to decrease tumor volume and the expression of Ki-67, as well as rationalize the potential need for future phase I and II clinical trials to be conducted to assess the potential of NK-1R antagonists, such as aprepitant, in osteosarcoma.
Figure 1. Combination of chemotherapy and aprepitant in osteosarcoma patients.
2. Role of NK-1R Antagonists as Antiangiogenic Agents in Osteosarcoma
Neoangiogenesis, with resultant endothelial cell proliferation, new vessel formation, and increased blood flow, is a hallmark of tumor development. Angiogenesis has been shown to play a critical role in osteosarcoma progression and metastasis [
5,
13]. SP and NK-1R have been found in the intra- and peritumor blood vessels in a large majority of tumors [
24]. It has also been reported that SP can directly stimulate the process of angiogenesis, through induction of endothelial cell proliferation. Two selective NK-1R antagonists have been shown to block SP-induced angiogenesis [
8,
25]. With specific relevance to osteosarcoma cells, aprepitant has been shown to decrease the expression of vascular endothelial growth factor (VEGF), a widely known mediator of angiogenesis [
13] (
Figure 1). Thus, the above findings suggest that aprepitant therapy can counteract tumor angiogenesis in osteosarcoma.
3. Role of NK-1R Antagonists as Agents That Counteract the Warburg Effect in Osteosarcoma
Glucose metabolism is strikingly different between normal cells and cancer cells. Production of ATP from glucose in normal cells leads to glucose oxidative phosphorylation via the TCA cycle. On the other hand, tumor cells convert glucose to lactate, which is known as the Warburg effect [
26]. This landmark discovery has been the subject of great interest and has resulted in the study of various agents that target glucose metabolism. The metabolic shift of glucose to lactate in cancer cells results in the generation of less ATP per glucose molecule metabolized. To compensate for this reduced energy and the need for higher energy by rapidly multiplying cancer cells, there is a need for increased rates of glucose uptake via potential overexpression of glucose transporters (GLUTs and SLC2 gene family). The GLUT1 protein and gene are overexpressed in osteosarcoma cells. Most importantly, high levels of GLUT1 are significantly associated with lymph node metastasis, age, and low survival rate [
27]. Furthermore, GLUT1 knockout studies have demonstrated significantly lower proliferation of OS cells in vitro and in vivo [
28,
29]. Moreover, in a nude mouse xenograft model of human osteosarcoma, the combination of adriamycin with a glycolytic inhibitor, 2-deoxy-D-glucose (2DG), resulted in significantly slower tumor growth (and, therefore, longer survival) compared to mice not treated with the glycolytic inhibitor [
30].
Tumor cells predominantly produce energy by means of a high rate of glycolysis followed by lactic acid fermentation; this is known as the Warburg effect. SP, in a concentration-dependent manner, promotes the breakdown of glycogen into glucose in glioma cells; resulting in higher glucose levels compared to normal cells. NK-1R antagonists in glioma cells prevent high levels of glucose from being formed and the ensuing glycolysis, thereby preventing the Warburg effect [
31,
32]. These important preceding studies indicated that NK1-R antagonists may serve as potential drugs that can counteract the Warburg effect (
Figure 1). Since osteosarcoma cells overexpress NK-1R, the use of an NK-1R antagonist, aprepitant, could counteract glucose production in osteosarcoma cells, thus preventing the Warburg effect in osteosarcoma cells. It is of tremendous importance that there are so far no drugs to counteract the survival effects of these tumor cells.
4. Role of NK-1R Antagonists as Antimetastatic Agents in Osteosarcoma
Osteosarcoma very often manifests with the invasion and metastasis of osteosarcoma tumor cells, leading to poor prognosis [
2]. Osteosarcoma metastasis may manifest during treatment of the primary tumor, shortly after treatment, or a long time after the end of the treatment [
2]. Studies have determined that aprepitant has the ability to inhibit the migrative ability of osteosarcoma tumor cells and reduce the levels of matrix metalloproteinases (MMP-2 and MMP-9), as well as NF-κB, a known stimulator of metastasis-related genes [
13]. Importantly, angiogenesis is one of the key factors leading to osteosarcoma progression and metastasis. Angiogenesis plays a critical role in osteosarcoma progression and metastasis. VEGF is the main angiogenic factor in tumor angiogenesis, supporting tumor growth and metastasis [
33]. It has been shown that VEGF and MMP-9 expression in osteolytic lesions of bone is associated with a higher risk of local recurrence and bone destruction [
34]. Aprepitant has the ability to reduce the levels of VEGF, thereby leading to reduced tumor metastasis [
13]. The antimetastatic effect of aprepitant in osteosarcoma is probably attributed to its ability to modulate the transcriptional regulator nuclear factor kappa B (NF-κB) and, subsequently, its target genes, including MMP-2, MMP-9, and VEGF-A [
13] (
Figure 1). It has been demonstrated that aprepitant at IC
50 concentrations of 31.55 μM and IC25 15.75 μM prevents migration of osteosarcoma cells in vitro by 80% and 42.5%, respectively. These results extrapolated to mg/kg are equivalent to approximately 20 or 10 mg/kg/day, respectively [
13]. It is a well-known fact that tumor cell migration is a prerequisite for invasion and metastasis; therefore, it is logical that the use of aprepitant can prevent invasion and metastasis in osteosarcoma patients. Patients with solid tumors very often need to undergo life-saving/prolongment surgical intervention for removal of tumor mass [
35,
36]. However, it is known that, if a surgical insult occurs or if a biopsy collection procedure is not performed correctly, it can very often precipitate or accelerate tumor recurrence and postoperative metastases [
35,
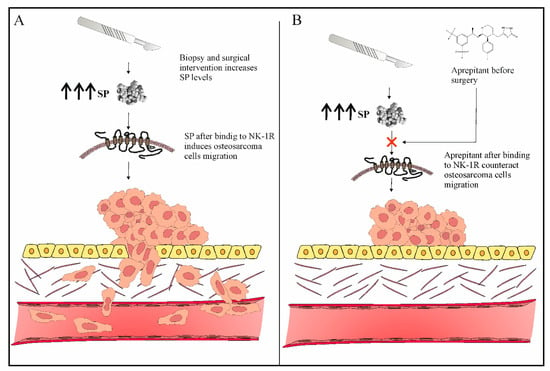
37]. Furthermore, one of the mechanisms via which the SP/NK-1R system may play a role in the pathogenesis of osteosarcoma is via SP-induced migration of osteosarcoma cancer cells (which are known to overexpress NK-1R). Therefore, if SP-induced neurogenic inflammation and pain following surgical intervention are not adequately addressed, the SP/NK-1R system may possibly play an important role in osteosarcoma pathogenesis. [
38]. Thus, it is crucial to find strategies aimed at reducing cancer recurrence and metastases after surgery. One of these strategies in osteosarcoma could be the use of aprepitant, a known inhibitor of invasion and metastasis of osteosarcoma cells before surgery [
13]. This strategy has never ever been tested; we suggest a 10 mg/kg (IC
25 15.75 μM) dose of aprepitant before surgical procedures (biopsy and surgical intervention), which has demonstrated an antimetastatic effect in osteosarcoma (
Figure 2). The preceding studies implicate that the use of aprepitant in osteosarcoma could possibly open the door for a new drug to be utilized in the prevention and treatment of osteosarcoma metastasis (
Figure 2).
Figure 2. (A) Surgical procedures (biopsy or surgical intervention) can induce osteosarcoma invasion and metastasis. (B) Aprepitant therapy before surgery can prevent osteosarcoma invasion and metastasis.
5. NK-1R Antagonists as Intelligent Drugs in Osteosarcoma Therapy
In the 21st century, the era of “molecularly targeted” anticancer therapy and of a “magic bullet” for cancer cells designed by Paul Erlich, NK-1R antagonists are new and promising anticancer drugs, which can be considered as a new types of “intelligent drugs” [
14,
47]. Conceptually, they go beyond other treatments because they display specific antitumor action. They inhibit tumor cell proliferation, induce apoptosis, and inhibit angiogenesis and migration of tumor cells for invasion and metastasis. In patients, they surprisingly provide other therapeutic benefits such as antinausea and anti-vomiting, as currently indicated and used in the clinic [
52]. Furthermore, the anti-inflammatory effect of aprepitant has been demonstrated in vitro and in vivo [
53], as well as in clinical trials [
49,
54]. Lastly, NK-1R antagonists were shown to serve as an antidepressant in clinical trials [
51], in addition to leading to hepatoprotection, neuroprotection, cardioprotection, and nephroprotection in in vitro and in vivo assays [
4]. Thus, aprepitant can be considered a potential therapeutic option for osteosarcoma (
Figure 1).
This entry is adapted from the peer-reviewed paper 10.3390/jcm12062135