As the first treatment targeting a human endogenous retrovirus (HERV) protein enters into phase III clinical trials to combat multiple sclerosis (MS) progression [
1], HERVs are becoming increasingly promising targets in cancer diagnostics and therapies. Previously, HERVs have been identified as involved in the progression of complex diseases such as MS [
1,
2], schizophrenia [
3,
4], and type 1 diabetes [
5,
6]. Lessons learned from these phenotypes have illuminated the potential for HERVs to impact human cancer development and treatment options. For example, there is currently a phase I trial testing the safety of a HERV-E-derived peptide autologous T-cell (HERV-E TCR T-cell) therapy to treat clear cell renal cell carcinoma [
7]. HERVs are single-stranded enveloped RNA viruses that integrated into the human germline by means of their long terminal repeats (LTRs) [
8]. Astonishingly, over 8% of the human genome comprise HERV sequences [
9,
10]. The LTRs in simple retroviruses, such as HERVs of the gammaretroviral subfamily, flank the capsid (
gag), polymerase and protease (
pol), and envelope (
env) genes. HERVs of the betaretroviral (e.g., HERV-K) or spumaviral (e.g., HERV-L) subfamily carry additional non-structural genes such as HERV-K
rec, HERV-K
np9 and HERV-L
tas/
bel1, and HERV-L
bet, respectively [
11]. In contrast to infectious retroviruses, about 87% of HERV sequences in the human genome are remnants of proviruses embodied by solo LTRs, while about 1.5% and 11.5% of HERV loci carry complete and truncated genomes, respectively [
12]. For this reason, HERVs were historically considered “junk” DNA [
13]. However, discoveries from the last two decades revealed substantial functions for various HERV elements in immune regulation [
14,
15,
16], cell differentiation [
17,
18], cell fusion [
19,
20], transcriptional regulation [
21,
22,
23,
24,
25], and cell transformation [
26,
27]. With this increasing evidence on the role of HERVs in cancer development, summaries of the current scientific knowledge are of high importance.
2. HERVs in Breast Cancer—The Rise of New Biomarkers
Breast cancer is the most common cancer and the leading cause of cancer-related deaths in women worldwide [
62]. Due to the many breast cancer subtypes and their varying treatment responses [
63], targeted treatments that evolved in recent years have become a success story. However, the field is still in need of preventive and early detection methods.
HERVs might be able to close this gap providing new targets for prognostics, diagnostics, and treatments. Several groups have independently reported the overexpression of messenger RNAs (mRNAs) and proteins from multiple HERV families in breast cancer cell lines and patient tissues compared to healthy tissues [
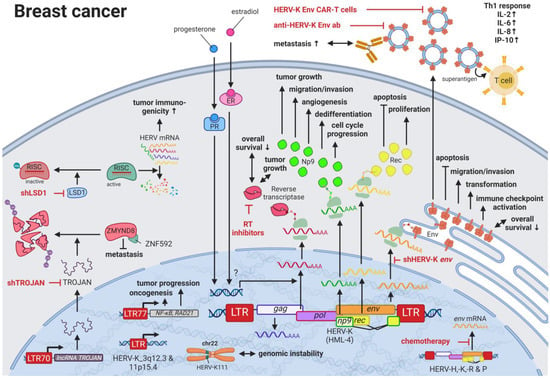
64]. Interestingly, the menstruation-associated hormones estradiol and progesterone were observed to increase HERV-K (HML-4)
env [
65] and HERV-K (HML-4) RT transcripts as well as HERV-K (HML-4) RT protein levels [
66] in breast cancer cell lines (
Figure 1). In breast cancer patients, increased HERV-K (HML-4) RT as well as HERV-K (HML-4) Env protein levels were shown to be associated with shorter metastasis-free and overall survival [
66,
67]. Conversely, Montesion et al. (2018) [
68] identified two HERV-K (HML-2) LTRs (HGCN:
ERVK-5 at position 3q12.3 and
ERVK3-4 at 11p15.4) that had specifically increased promoter activity in breast cancer while decreased activity in immortalized human mammary epithelial cells. Additionally, several stage-specific transcription factor (TF)-binding sites within the two LTRs were predicted to potentially contribute to promoter activity during neoplasia [
68]. While the
ERVK-5 (HERV-KII) was fixed in humans, the
ERVK3-4 (HERV-K7) was found to be polymorphic in the human population with an allele frequency of 51%, presenting the prospect of a newly identified risk facto r [
68]. In addition, breast cancer cell lines were shown to harbor HERV-K111 gene conversion/deletion events in the pericentromeric region of chromosome 22, suggesting a contribution to genomic instability [
69].
Figure 1. The role of HERVs in breast cancer. Evaluated treatments are marked in red. Abbreviations: Th1 = T helper cell 1, ER = estradiol receptor, PR = progesterone receptor, LTR = long terminal repeat, gag = group antigen (capsid), pol = polymerase, RT = reverse transcriptase, env = envelope. If not otherwise stated HERV-K = HML-2.
Higher expression of particular HERV-K gene products in patients with breast cancer is also signified by the pronounced immune response against such proteins. Responses include increased T-cell proliferation, Th1-specific cytokine secretion (i.e., INFγ, IL2, IL6, CXCL8, CXCL10), immune checkpoint activation, and serum antibody production against HERV-K (HML-2) proteins [
70,
71,
72]. Additional to higher serum HERV-K mRNA levels and serum anti-HERV-K antibody titers in women with ductal carcinoma in situ and stage I disease compared to women without cancer, Wang-Johanning et al. (2014) reported that elevated HERV-K (HML-2) antibodies and mRNA levels in the blood can be an early indicator of future metastatic disease development [
72]. In patients undergoing chemotherapy, HERV-H, -K, -R, and -P
env mRNA expression is reported to be decreased compared to patients not receiving chemotherapy [
73].
A detailed study of the HERV-K (HML-2)
env knockdown through RNAi revealed the involvement of the viral gene in cellular pathways playing key roles in cancer (e.g.,
EGFR,
TGF-β,
NF-κB,
MYC,
p53,
HRAS,
KRAS, and
MAPK1/3) (
Figure 2) [
75]. Overexpression of HERV-K (HML-2)
env, on the other hand, increased breast cancer cell transformation, migration, and invasion, as well as restored the cancer-related signaling pathways mentioned above alongside the downregulation of p53 (HGNC: TP53) [
75]. Additionally, microarrays identified HERV-K (HML-2) Env protein as a strong inducer of the MAPK pathway via upstream TFs [
77], and examinations of the DNA methylome and TF-binding data revealed several HERV LTR77-driven TFs such as NF-κB (HGNC: NFKB1) and RAD21 [
41,
78]. Besides HERV-K (HML-2) Env, HERV-K (HML-2) non-structural nuclear protein Np9 has been described to interact with cellular proteins [
79]. Np9 destabilizes LNX1, an E3 ubiquitin ligase, that targets members of the NUMB/NOTCH1 pathway for degradation [
79,
80]. NOTCH1 regulates cell differentiation, cellular metabolism, cell cycle progression, angiogenesis, self-renewal, and immune function [
81] and has been shown to be deregulated in breast cancer [
82].
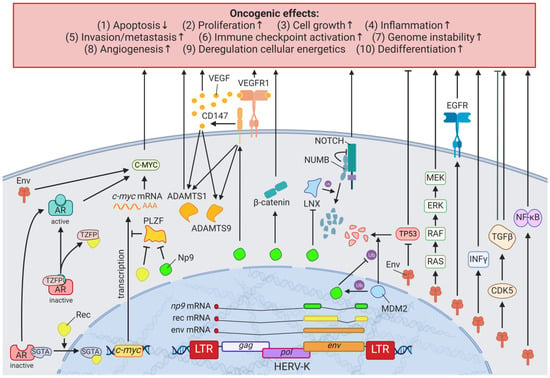
Figure 2. The effects of HERV-K (HML-2) Np9, Rec, and Env proteins on oncogenesis. LTR = long terminal repeat, gag = group antigen (capsid), pol = polymerase, env = envelope, HERV-K = HML-2.
3. HERVs in Lymphoma—The Silent Inducers
Lymphomas are characterized by an increased proliferation of lymphocytes and are classified according to their maturity (peripheral or mature versus precursor) and cell lineage (B, T, or natural killer cell) [
91]. In contrast to other cancers, a hallmark of lymphoma is its origin in the immune system, with known risk factors that perturb immune functions, such as immunosuppressive drugs [
92], autoimmune disorders [
93], and viral infections including Epstein–Barr virus (EBV), human immunodeficiency virus (HIV), or human T-cell lymphotropic virus (HTLV) [
94]. As there is complex interaction between HERVs and immune system function, it is consistent with previous literature that HERV deregulation influences lymphomagenesis.
HERV-K (HML-2) was shown to have markedly different titers in the blood of patients with lymphoma (e.g., HIV infection with diffuse large B-cell lymphoma (DLBCL), non-HIV diffuse large B-cell lymphoma, and HIV infection with Hodgkin lymphoma (HL)) compared to healthy individuals [
95]. Remission of the cancer after successful treatment was associated with a significant decrease in viral titers [
95]. While there was a large range of titer differences between patients with lymphoma (on average 10
10 copies/mL) and healthy individuals (on average 10
2 copies/mL), HERV-K viral particles were found in the plasma of all patients with lymphoma [
95].
Contrary to the pathogenic effects of HERVs described above, the double-copy HERV-R on chromosomes 7q11.21 and 7q33 (HERV-R.3-1 and HERV-R.3-2, respectively) has been classified to have tumor suppressive functions [
107]. HERV-R.3-1 Env (HGNC: ERV3-1
ε) was observed to be downregulated in HL cells compared to normal blood cells [
107], which parallels the absence of expression seen in choriocarcinoma [
108]. In choriocarcinoma cells,
ERV3-1 overexpression inhibits cell proliferation [
109], while ERV3-1 is upregulated during terminal differentiation of leukemia cells and is highest in cell cycle arrested cells [
107,
110,
111]. Downregulation of cyclin B and upregulation of the cyclin-dependent kinase inhibitor P21 (HGNC: CDKN1A) are likely to be key mechanisms for growth inhibition [
109].
Besides cancer pathways in which HERVs have incorporated themselves, advances in treatments have revealed HERVs being part of therapy-related side effects and drug resistance mechanisms. ABCB1 (MDR-1) is one of the most expansively studied drug resistance mechanisms [
114]. The
ABCB1 gene encodes a 170-kDa ATP-dependent efflux pump for the plasma membrane, which prevents intracellular drug accumulation [
114]. Interestingly, aberrant MDR-1 transcription found in lymphoma cells is driven by the ERV1 LTR MER57 initiating transcription in the opposite direction of the viral promoter [
114]. To reduce the transcription of detrimental genes, several DNA methyltransferases (DNMT) and histone deacetylase (HDAC) inhibitors have been incorporated in the treatment plan for hematopoietic and lymphatic malignancies in recent years [
115].
4. HERVs in Leukemia—The Lifesavers for Cancer Cells
Leukemias are the most common childhood cancers worldwide [
62,
121] and among the cancers with the lowest somatic mutational burden [
122]. Both characteristics suggest genomic risk factors that can be inherited, and when accumulated, lead to carcinogenesis. Analogous to observations made for HERV LTRs in prostate cancer (see HERVs in Prostate Cancer—The Dancing Partner of the Androgen Receptor), THE-7 LTRs were discovered as drivers of a translocation of chromosome 14q32 to chromosome 7q21 in a female patient with B-cell chronic lymphocytic leukemia (B-CLL) [
123]. Furthermore, fibroblast growth factor receptor 1 (
FGFR1) was found to be constitutively activated through the fusion between a HERV-K3 (HML-6) sequence (HGCN: ERVK3-1) and the
FGFR1 gene in a male patient with an atypical stem cell myeloproliferative disorder [
124,
125]. The fusion and resulting aberrant growth signal were the result of a translocation involving chromosomes 19q13.3 and chromosome 8q12 [
124,
125]. Furthermore, deletions of pericentromeric HERV-K111 regions in adult T-cell leukemia cell lines were enriched leading to chromosomal instabilities [
69]. Additional to these chromosomal abnormalities as prognostic markers, Schmidt et al., (2015) identified single nucleotide polymorphism (SNP) markers near two endogenous retroviral loci, HERV-K (HML-2) on chromosome 1 (HGCN:
ERVK-7) and HERV-Fc1 on chromosome X (HGCN:
ERVFC1) associated with multiple myeloma [
126]. Both HERV regions encode nearly complete viral proteins, suggesting a functional involvement of the gene products in disease development [
126].
Similar to the observations in lymphomas, the immune response against HERV-K (HML-2) and other HERVs appears to be rather weak or unexplored (see also HERVs in Lymphoma—The Silent Inducers) [
100]. This might also be due to the fact that HERV-K108 (HGCN: ERVK-6) Env TM has immunosuppressive properties and has been reported to induce IL10 in PBMCs [
127]. IL10 is an anti-inflammatory cytokine, which terminates T-cell responses and leads to immune tolerance [
128]. In a comparable way, surface
CD5 expression on B cells regulates their functional fate and immunological activity.
CD5 expression is tightly controlled through a HERV-E sequence located upstream of the
CD5 locus (
HERV-E::CD5). The
HERV-E::CD5 sequence was shown to induce the integration of an alternate exon, resulting in low levels of membrane CD5 in normal B cells [
129].
5. HERVs in Skin Cancer—The Highly Addictive Treatment Targets
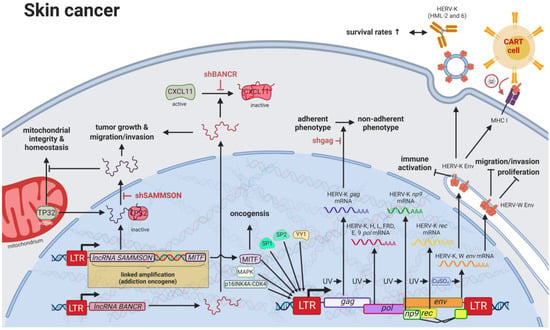
Compared to other organs, the skin is exposed to some of the highest amounts of mutagens; therefore, skin cancer is the malignancy with the highest mutational burden [
122]. Accordingly, several physical and chemical agents with mutagenic potential have been proven to influence the regulation of HERV sequences [
9,
10]. As such, UV radiation, the primary risk factor for both melanomas and non-epithelial skin cancers, was shown to induce
gag expression of HERV-K (HML-2) [
143] in melanoma cell lines and tumor tissues; to reduce
rec and
np9 expression of HERV-K (HML-2) in primary human melanocytes and melanoma [
144]; and to reduce
pol expression of HERV-K, -H, -L, -FRD, -E, and ERV9 in melanoma cell lines [
145], primary keratinocytes [
146], and skin biopsies (
Figure 4) [
147].
Figure 4. The role of HERVs in skin cancer. Evaluated treatments are marked in red. Abbreviations: LTR = long terminal repeat, gag = group antigen (capsid), pol = polymerase, RT = reverse transcriptase, env = envelope, lncRNA = long non-coding RNA, shBANCR = siRNA targeting BANCR. If not otherwise stated HERV-K = HML-2.
Besides HERVs, many oncogenes have been found in higher levels in skin cancers, likely caused by mutated TF-binding sites in promoters [
151]. HERV LTRs have been postulated to harbor over 64% of all human-specific TF-binding sites in human embryonic stem cells [
152], and chromatin immunoprecipitation (ChIP) assays as well as gene expression studies revealed that one third of p53 (HGCN: TP53) sites are located within HERV LTRs [
153]. Of all LTR-associated p53-binding sites, approximately 70–90% are located in sequences of the ERV1 family [
154], while HERV-I LTRs have been shown to be repressed by TP53 and activated by TP53 mutations [
155].
Despite the general assumption that the majority of HERV sequences in the genome are inactive due to DNA methylation and other histone marks, a study published by Jacques et al. (2013) indicated that up to 80% of HERV regions exist in an open chromatin state [
159]. Particularly in patients with melanoma, both lncRNAs,
BANCR and
SAMMSON, are promoted by HERV LTRs [
49,
160,
161]. Both lncRNAs were confirmed to increase growth and invasiveness of melanocytes [
160,
161].
6. HERVs in Testicular Cancer—The Governors of Tumor Suppressor Genes
Although the secretion of HERV viral particles was first detected in placenta by Kalter et al. (1973) [
175], three years later, testicular tumors became the first cancer tissue described to selectively release HERV viral particles [
176]. Since then, testicular cancer cell lines serve as a model for HERV-K particle assembly and the effects of HERV-K protein expression on cell functions [
177,
178,
179]. Cellular transcription factor YY1 was shown to specifically activate LTRs in teratocarcinoma cell lines [
180] with the HERV-K (HML-2) LTR in Tera-1 cells becoming as strong of a promoter as the SV40 early promoter (
Figure 5) [
181].
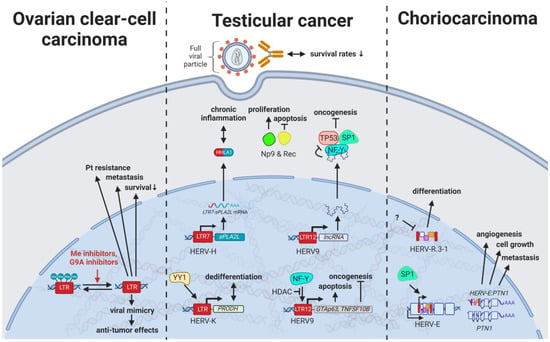
Figure 5. The role of HERVs in genital cancers. Evaluated treatments are marked in red. Abbreviations: HDAC = histone deacetylases, Me = methylases, G9A = G9a methyltransferase, Pt = platinum treatment, HERV-K = HML-2.
With HERV viral particles being secreted by testicular tumor cells, over 60% of males with germ cell cancers have been observed to display seroreactivity to HERV-K (HML-2) antigens, while only 4% of healthy individuals showed high antibody titers [
177]. Additionally, HERV-K(HML-2)-specific T cells could be detected in patients with a history of testicular cancer, indicating immunological memory formation that may prevent relapse in certain cases [
185].
Specifically, the two viral proteins derived from alternative transcripts of the C-terminal portion of the HERV-K (HML-2)
env gene were first discovered in GCT cell lines [
187] and subsequently described to play a central role in the development of GCTs [
188]. The proteins, later termed HERV-K (HML-2) Np9 [
130] and Rec [
189], associate in GCTs with the tumor suppressor PLZF (HGNC: ZBTB16), a transcriptional repressor, and chromatin remodeler and abolish its transcriptional repression of
MYC, a major target of PLZF [
190]. This leads to amplified cell proliferation and survival mediated by overexpression of
MYC and corresponding MYC-regulated genes [
190].
7. HERVs in Other Genital Cancers (Ovary Cancer, Choriocarcinoma, and Endometrial Cancer)—The Ascent of New Possibilities
Despite stably falling incidences of over the last three decades [
197], most patients with ovarian cancer are not diagnosed until stages III (51%) and IV (29%) because they experience few or no symptoms until the disease has metastasized [
198]. Therefore, HERVs have been investigated in different subtypes for their potential as prognostic, diagnostic, and therapy-resistance markers. Heidmann et al. (2017) proposed HEMO, a HERV MER34-derived Env protein, as a possible marker for ovarian clear-cell carcinoma (OCCC) as it was significantly increased in ovarian cancers with evidence for histiotype dependence [
199]. In a similar way, HERV-W, HERV-E, and HERV-K (HML-2) displayed higher expression due to generalized hypomethylation in ovarian carcinomas compared to non-malignant ovarian tissues [
200,
201,
202]. Intriguingly, hypomethylated HERV-K (HML-2) elements in OCCC were observed to be associated with poor prognosis, platinum-based therapy resistance, and increased metastasis (
Figure 5) [
201].
ERVW-1
ε and ERVFRD-1
ε are the most extensively studied retroviral proteins encoded in the human genome and have a vital role in placentation by facilitating trophoblast fusion and creation of an immune privileged site [
20,
40]. Nonetheless, HERVs have been implicated in furthering abnormal growth and reduced differentiation of trophoblasts resulting in the development of choriocarcinoma. HERV-E induces expression of an alternative transcript of
PTN, a heparin-binding protein with central functions in growth and differentiation control of the placenta [
207,
208,
209]. The alternative
HERV-E:PTNλγπελθ transcript is driven by the TF SP1 binding to the HERV-E LTR in untranslated exon 1 of PTN [
209].
HERV-E:PTNλγπελθ mRNA was only detected in trophoblast cell cultures while absent in normal adult tissues (
Figure 5) [
208,
209].
8. HERVs in Colorectal and Gastrointestinal Cancers—The Hopes and Hazards of Family H
Colorectal cancers (CRCs) are among the most common cancers worldwide with strikingly low 5-year survival rates of less than 65–70% in Northern America, Australia/New Zealand, and many European countries [
62]. While early diagnosis has markedly improved for older patients due to routine screenings in individuals >50 years of age, rising rates in the population under 45 years of age highlight the need for improved non-invasive prognostic and diagnostic tools [
213]. In the last decade, research has started to focus on the influence of HERV elements on CRCs and has revealed interesting interactions, especially involving HERV-H. With over 1000 loci in the human genome, HERV-H is the most abundant HERV family carrying coding regions in the human genome [
214].
A link between chronic inflammation and cancer has been established for various tumors, and, specifically for CRCs, an inflammatory microenvironment has been recognized to be a cause, hallmark, and consequence of disease [
221]. A subset of patients with colon cancer was shown to express a
HERV-H_9q24.1::IL33 fusion transcript required for tumor growth [
222]. IL33 is a proinflammatory cytokine produced by epithelial and endothelial cells [
223] that has been demonstrated to correlate in its expression with CRC progression and metastasis [
224]. The particular function of the
HERV-H_9q24.1::IL33 product is unknown but, based on the function of native IL33, might include roles as a modified cytokine or nuclear factor regulating gene transcription [
223]. Further, HERV LTR promoted chimeric transcripts detected specifically in CRC tissues and cell lines including the ion transporter
SLCO1B3λπ, which is frequently mutated in CRC [
222].
In contrast to fusion transcripts that result in aberrant cellular genes, TIP60 (HGNC: KAT5) has been described as a regulator of the inflammatory effects of HERV expression inside the cell [
225]. KAT5 is a tumor suppressor that is found to be repressed in early stages of CRCs and breast cancers [
225]. A publication by Rajagopalan et al. (2018) indicated that KAT5 downregulation results in increased levels of HERV expression and associated inflammatory responses [
225]. In normal cells, KAT5 induces H3K9 trimethylating enzymes SUV39H1 and SETDB1 in a BRD4-dependent manner, which leads to global inhibitory methylation of HERV loci [
225]. In KAT5-repressed cancer cells, the study investigators detected induction of IRF7 mediated by the intracellular pathogen sensing STING (HGNC: TMEM173), resulting in an inflammatory response and further tumor growth [
225].
While viral gene products are readily detected by innate immune receptors, most cellular lncRNAs escape the surveillance mechanisms and, in this fashion, are able to interfere with regulatory pathways. For instance, the lncRNA
EVADR on chromosome 6q13 was observed to be induced by the ERV1 LTR MER48 specifically in colon, rectal, lung, pancreas, and stomach adenocarcinomas [
226]. In a similar way, the ERV1 LTR MER61C on chromosome 514.1 drives transcription of the lncRNA
PURPL (
LINC01021), which is increased in CRC cell lines and tumors [
227,
228]. While higher
EVADR expression was associated with slightly decreased patient survival rates [
226], CRC tumors with higher levels of
PURPL RNA resulted in improved survival rates, and induced expression in CRC cell lines lead to increased chemosensitivity according to Kaller et al. (2017) [
227].
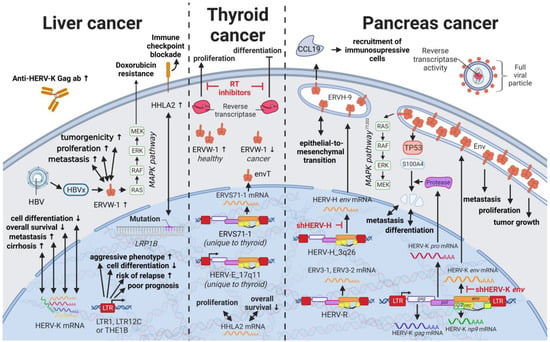
9. HERVs in Liver Cancer—The Opening Chapter
Liver cancers are the third leading cause of cancer-related death in males worldwide [
235]; however, only limited reports are available on the influence of HERVs in liver cancer oncogenesis. Ahn and Kim (2009) together with Liang et al. (2009) reported increased expression of HERV-H, HERV-R.3-1, and HERV-P in overall liver cancers without taking into consideration distinct cancer subtypes [
236,
237], while several other groups reported the distinct activation of HERV-K (HML-2) and HERV-P in hepatocellular carcinomas (HCCs) specifically [
238,
239]. However, a study by Liu et al. (2021) demonstrated that the LRP1B mutation was associated with the overexpression of HERV-H LTR-Associating 2 (HHLA2) in patients with HCC [
231]. HCC has a high morbidity and constitutes more than three-fourths of all cases with liver cancer [
62]. Interestingly, a large number of HERV LTRs, including LTR1, LTR12C, and THE1B, were found upregulated in human HCC tumors (
Figure 7) [
240]. HCC tumors with high LTR activation were associated with high risk of relapse [
240,
241], a more aggressive phenotype [
240,
241], poor prognosis [
241], and impaired cell differentiation in animal models [
16,
242]. Moreover, HERV-K (HML-2) gene products, while only moderately elevated in patients [
243], were described to positively correlate in their expression with tumor cell dedifferentiation, mortality rates, TNM stage, and cirrhosis in HCCs [
239]. Furthermore, in a subgroup of patients with HCC antibodies against HERV-K (HML-2) Gag were found indicating the immunogenicity of the viral gene product [
238,
243]. Additional to its potential as prognostic marker and therapeutic target, HERV-K (HML-2) expression might also provide resolution to a dispute concerning the origin of a liver cancer cell line. It is still debated whether HepG2 cells are derived from HCC or hepatoblastoma.
Figure 7. The role of HERVs in liver and endocrine cancers. Evaluated treatments are marked in red. Abbreviations: HBV = hepatitis B virus, MAPK = MAP kinase, ab = antibody, LTR = long terminal repeat, gag = group antigen (capsid), pol = polymerase, RT = reverse transcriptase, env = envelope, HERV-K = HML-2.
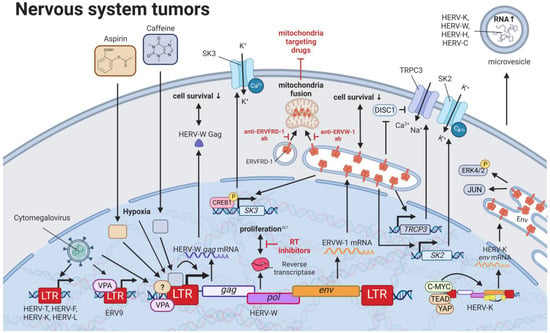
10. HERVs in Nervous System Cancers—The Wicked Side of HERV-W
Perhaps the best described and most widely accepted involvement of HERVs in pathogenesis has been in neurological diseases. Links between HERVs and MS, amyotrophic lateral sclerosis, and schizophrenia are supported by numerous publications and have been summarized well by Gruchot et al. (2019) [
249] and Dolei et al. (2019) [
250]. Interestingly, one specific HERV-K (HML-2)-derived SINE fragment was identified by random amplified polymorphic DNA to be absent in a patient with a grade IV glioblastoma (GBM) [
251]. Even though this finding was unique to the patient and could not be observed in 32 other gliomas, the method might be useful to identify the absence of HERV-K (HML-2) sequences normally located in tumor suppressor genes, such as
BRCA2,
XRCC1, and
NBPFs, as a marker for oncogenesis (
Figure 8) [
251,
252]. On the contrary, a much more pronounced role in inflammatory neurological disease as well as brain cancers has been confirmed for HERV-W. Substances such as caffeine and aspirin that are able to pass the blood–brain barrier have been reported to increase ERVW-1
ε protein and HERV-W
Gag mRNA as well as protein levels in human SH-SY5Y neuroblastoma cells [
253]. The concentration for both substances correlated positively with HERV-W gene expression and negatively with cell survival [
253]. Intriguingly, caffeine acted in a luciferase assay on the HERV-W promoter, while aspirin did not change the promoter activity [
253].
Figure 8. The role of HERVs in nervous system tumors. Evaluated treatments are marked in red. Abbreviations: K+ = potassium, Ca2+ = calcium, ab = antibody, LTR = long terminal repeat, gag = group antigen (capsid), pol = polymerase, RT = reverse transcriptase, env = envelope, HERV-K = HML-2.
Through studies of neuroblastoma cell lines SH-SY5Y and IMR-32, ERVW-1
ε was demonstrated to stimulate the expression of
SK3 [
263] and
TRPC3 [
264]. Additionally, ERVW-1
ε-mediated SK3 channel activation was identified to be dependent on the CREB1 and documented to result in an increased potassium ion (K
+) current [
263]. On the other hand, the TRPC3 channel was postulated to be activated through either the derepression of the TRPC3 inhibitor,
DISC1, or the direct induction of
TRPC3 expression by ERVW-1
ε [
264].
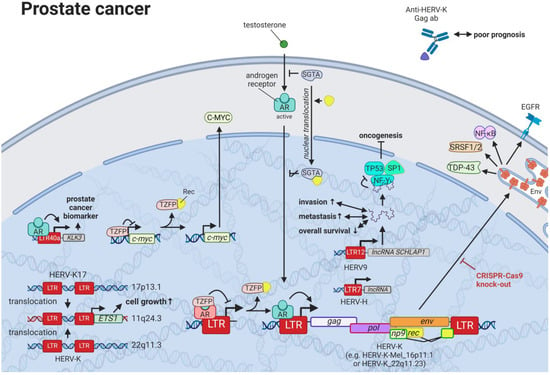
11. HERVs in Prostate Cancer—The Dancing Partner of the Androgen Receptor
Prostate cancer is the most frequent cancer in males in Western countries and the second most common cancer in males worldwide [
62]. Despite increased survival rates, curative treatments, such as surgery and radiation, convey serious side effects [
62], so that active surveillance becomes the preferred approach for men with less-aggressive prostate cancer. Directed therapies targeting HERVs that are reportedly dysregulated in prostate cancer cell lines and tissues present a milder approach for prostate cancer treatment. A prime candidate in prostate cancer is HERV-K, which exhibits tight interactions with the testosterone receptor. While certain HERV-Ks, such as HERV-K17 on chromosome 17p13.1 (not HGCN: ERVK-17), show tissue-specific upregulation in prostate cells and downregulation in malignant cells [
271,
272], other members of the family, such as HERV-K-Mel (HML-6) on chromosome 16p11.1 [
273] and HERV-K (HML-2) on chromosome 22q11.23 [
238,
274], display significantly higher expression in prostate cancer tissues and cell lines compared to healthy controls (
Figure 9). This increased expression was identified to be androgen-dependent with several HERV-K (HML-2) LTRs containing predicted steroid hormone receptor-binding sites [
274]. HERV-L LTR40a was shown to exhibit ligand-dependent recruitment of the androgen receptor (AR) functioning as an enhancer for
KLK3 [
275]. KLK3 is the most well-studied biomarker for prostate cancer and is a model system to study androgen signaling [
276]. As prostate cancer cell survival is highly dependent upon AR signaling, LTR40a induction might provide an alternative marker for disease detection. Additionally, inhibition of the LTR40a and its associated gene products could prevent the synthesis of HERV-regulated oncogenes. Reciprocal to the activation of HERV LTRs by the AR, HERV-K (HML-2) Rec has been documented to associate with the AR co-repressor, TZFP (HGNC: ZBTB32), relieving the TZFP-mediated repression of AR-induced transactivation [
277].
Figure 9. The role of HERVs in prostate cancer. Evaluated treatments are marked in red. Abbreviations: AR = androgen receptor, ab = antibody, lncRNA = long non-coding RNA, LTR = long terminal repeat, gag = group antigen (capsid), pol = polymerase, RT = reverse transcriptase, env = envelope, HERV-K = HML-2.
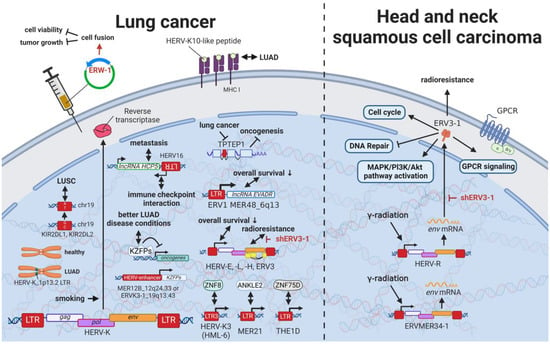
12. HERVs in Lung Cancer—The Love for Long Noncoding RNAs and Pseudogenes
Even though lung cancers are by far the deadliest cancers worldwide [
62] and carry an equally high mutational burden as melanomas [
122], only limited research on the relationship between HERVs and pulmonary diseases has been published. Being one of the leading causes of mutations in lung cancer, smoking was shown to induce HERV-K (HML-2 and 6)
pol expression in multiple tissues (
Figure 10) [
287,
288]. Insertional polymorphisms (absence and presence of a HERV sequences at a specific locus) can alternatively lead to differences in numerous regulatory elements at a specific locus and therefore have gained increased interest in the research community. Kayho et al. (2013) were able to demonstrate that HERV-K (HML-2)_soloLTR(1p13.2) homozygosity in never-smoker women is statistically associated with increased susceptibility to lung adenocarcinoma [
288]. This finding is supported by the observation of a peptide-carrying sequence homology with HERV-K10 LTR specifically expressed in human lung adenocarcinoma A549 and absent in non-transformed fibroblasts [
289]. The Krüppel-associated box domain-containing zinc-finger family protein (KZFP) is a transcriptional suppressor, which when expressed in cancer cells alters the expression of genes expression of genes related to the cell cycle and cell-matrix adhesion and suppresses cellular growth, migration, and invasion abilities [
290].
Figure 10. The role of HERVs in lung, head, and neck cancer. Evaluated treatments are marked in red. Abbreviations: LUAD = lung adenocarcinoma, LUSC = lung squamous cell carcinoma, GPCR = G-protein-coupled receptor, LTR = long terminal repeat, gag = group antigen (capsid), pol = polymerase, env = envelope. If not otherwise stated HERV-K = HML-2.
Pan-cancer analysis revealed an association between HERV and KZFP expression [
290]. In particular, CRISCR-Cas9-mediated knockout of the HERV-enhancer 1 on chromosome 12q24.33 (MER21B) and HERV-enhancer 2 on chromosome 19q13.43 (HERVK3-int, HGCN: ERVK3-1) resulted in decreased KZFP expression in lung adenocarcinoma (LUAD) cancer cell line A549 [
290]. Overall increased KZFP expression was associated with better prognosis and lower cancer stage in patients with LUAD [
290], while another study demonstrated less favorable outcomes linked to increased HERV—in particular HERV-E, HERV-L, HERV-H, and ERV3 expression [
291].
13. HERVs in Cancers of the Urinary System (Kidney and Bladder Cancer)—The Future Fire Fighters
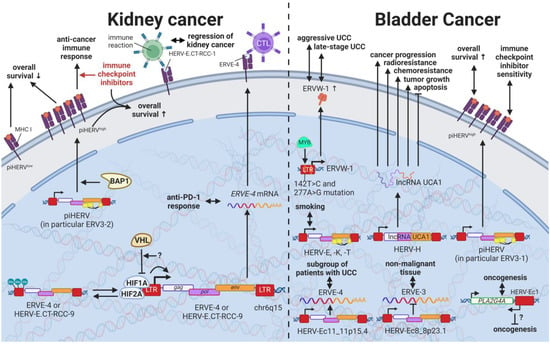
While most HERVs operate below immune detection, research has shown that upregulation of HERVs in transformed cells can serve as a physiological tumor recognition signal, preventing the propagation of cancerous cells in early stages [
117,
140,
203,
230]. In advanced-stage cancers, such tumor suppressive functions are disrupted on multiple levels, one being through immune checkpoint activation [
299]. Hence, newly developed immune checkpoint inhibitors have proven to be effective regimens for persistent cancers, especially for clear cell renal cell carcinoma (ccRCC), where clinically significant and robust responses have been observed [
300]. To further enhance antitumor immune responses upon immune checkpoint blockade, Panda et al. (2018) examined HERVs as prospective inducible targets in patients with ccRCC [
71]. The study investigators determined the subset of potentially immunogenic HERVs (piHERVs) with the greatest potential to induce immune responses, such as immune infiltration, higher cytotoxic T-cell levels, and M1 macrophage abundance (
Figure 11) [
71]. Despite lower overall survival of patients with higher expression of such HERVs, piHERV
high patients treated with immune checkpoint inhibitors experienced significantly improved prognosis and treatment responsiveness compared to their piHERV
low counterparts [
71]. Out of all piHERVs, HERV-R.3-2
env (
ERV3-2) expression was particularly increased in responders compared to non-responders, highlighting its tumor suppressive functions mentioned for other cancers (see HERVs in Lymphoma—The Silent Inducers and HERVs in Other Genital Cancers (Ovary Cancer, Choriocarcinoma, and Endometrial Cancer)—The Ascent of New Possibilities) [
71]. Interestingly, similar results have been observed for patients with urothelial cancer who displayed high piHERV expression [
301].
Figure 11. The role of HERVs in cancers of the urinary system. Evaluated treatments are marked in red. Abbreviations: CTL = cytotoxic T cell, UCC = urothelial carcinoma, RCC = renal cell carcinoma, piHERV = potentially immunogenic HERVs, LTR = long terminal repeat, gag = group antigen (capsid), pol = polymerase, env = envelope, HERV-K = HML-2.
Several underlying mechanisms have been proposed to drive the inherently higher expression of piHERVs. On the genetic level, piHERV
high patients demonstrated an enrichment of mutations in the
BAP1 gene, which is a deubiquitinase known to functionally associate with chromatin modulators [
71,
302]. The functional disruption of BAP1 may thus lead to chromatin remodeling resulting in piHERV expression [
71]. A subgroup of patients with ccRCC was found to express HERV-E in high amounts driven by a hypomethylated LTR [
303]. Inactivation of the von Hippel–Lindau tumor suppressor gene (
VHL) with subsequent stabilization of hypoxia-inducible transcription factors, HIF1A and -2A, was shown to induce expression of two HERV-E transcripts on chromosome 6q15 (
HERV-E.CT-RCC-8 (HGCN: ERVE-4) and
HERV-E.CT-RCC-9) through the HIF response element located in the viral LTR [
303]. In other tumors and matched normal tissues, a hypermethylated LTR was reported to prevent the induction of the HERV-E.CT-RCCs [
303].
14. HERVs in Endocrine Cancers (Pancreas and Thyroid Cancer)—The Unknown Potential
Despite their rarity, endocrine tumors, particularly thyroid cancers, have dramatically increased in their incidence worldwide in the last four decades [
316,
317]. Additionally, anaplastic thyroid cancer [
318] and adrenocortical carcinoma [
319] have incredibly short survival times due to their aggressive nature, while pancreatic ductal adenocarcinoma is often associated with increased risk for metastasis and high mortality rates due to a lack of symptoms in early stages [
320,
321]. In pancreatic cancer, HERV-K (HML-2) has been suggested as a major tumor driver. Li et al. (2017) reported HERV-K (HML-2)
env,
gag, and
np9 genes as well as HERV-K (HML-2) Env protein expression to be significantly increased in pancreatic cancer cell lines as well as patients with pancreatic cancer compared to healthy controls [
322]. The RNAi-mediated knockdown of HERV-K (HML-2)
env reduced proliferation and colony formation of the cancer cell lines and resulted in decreased tumor growth and metastasis in an in vivo mouse model [
322]. Pathway analysis by Li et al. revealed an activation of the RAS/MEK/ERK pathway and inhibition of TP53 by HERV-K (HML-2) in pancreatic cancer cells [
322], which was confirmed in breast cancer cells by Lemaître et al. (2017) (see Results HERVs in Breast Cancer—The Rise of New Biomarkers) [
77]. Furthermore, the cleavage of S100A4 by the HERV-K (HML-2) protease was associated with cell cycle progression, differentiation, and metastasis in pancreatic cancer [
323].
In addition to HERV-K (HML-2) Env, HERV-H Env was described to contribute to the oncogenesis of pancreatic cancers. HERV-H on chromosome 3q26 is one of the few from the family that contain a complete open reading frame for the Env protein (HGCN: ERVH-9
ε) [
324]. This ERVH-9
ε protein, also called Env60, has been upregulated in pancreatic cancer cells undergoing epithelial-to-mesenchymal transition (EMT), while also expressed in healthy pancreatic tissue [
325]. Particularly, the immunosuppressive portion of the ERVH-9
ε protein amplified EMT and induced
CCL19 expression, which significantly correlated with the recruitment of immunosuppressive cells in patients [
325].
15. HERVs in Other Cancers (Osteosarcoma, Head and Neck Squamous Cell Carcinoma)—The Hodgepodge of Hope for Novel Therapies
Rare cancers, by definition, only provide limited case numbers for investigation. Accordingly, only single reports of HERVs evaluated in such cancers are available. Despite a high incidence in children, osteosarcoma is a rare malignancy in adults [
337]. A single study on human osteosarcoma reported the statistically significant upregulation of 35 and downregulation of 47 HERV mRNAs in osteosarcoma tissues compared to healthy controls [
337]. The most significant HERV elements differentially expressed included LTRs of the HERV-L, HERV-K (HML-2), and ERV-1 [
337].
Head and neck squamous cell carcinomas (HNSCC) comprise 90% of all head and neck cancers and are relatively common [
341,
342]. HNSCCs are often inoperable due to the complex anatomy, making radio- and chemotherapy the only option [
343]. Accordingly, radioresistance poses a major problem resulting in very low survival rates [
62,
343]. Findings by Michna et al. (2016) documenting an induction of
ERV3-1 and ERVMER34-1
env upon exposure of HNSCC cell lines to γ-radiation indicate a potential target to overcome radioresistance [
341].
16. Novel Options for Cancer Treatment Facilitated by HERVs
Many studies have demonstrated increased HERV levels in tumor cell lines and tumor tissues compared to normal healthy tissues, suggesting two potential treatment approaches. On the one hand, strategies have been proposed that target pathways in which HERVs are involved [234,345,346], as outlined for various cancers above. HERVs might provide another pharmacological target in this way. Moreover, HERV-derived HERV restriction factors such as suppressyn (HGCN: ERVH48-1), a HERV-F-derived inhibitor of ERVW-1ε-mediated fusion, might serve as a starting point for drug development. However, discoveries of HERV genes and LTRs involved in regulatory mechanisms are very new and still advancing with the recent development of more accurate and affordable sequencing techniques.
On the other hand, treatment strategies targeting HERV proteins as tumor-specific antigens have been suggested [83,84], assuming HERV expression is a consequence of transcriptional changes in tumors. HERVs as cancer-specific antigens in hematological cancers appear to be particularly promising. Saini et al. (2020) found HERV-specific T cells are present in 17 of the 34 patients with leukemia, recognizing 29 HERV-derived peptides representing 18 different HERV loci, among which ERVH-5, ERVW-1, and ERVE-3 had the strongest responses [347]. Furthermore, the ancestral retroviral HEMO envelope gene (Human Endogenous MER34 ORF) is hailed as a pan-cancer target for leukemia, lung, adrenal, thyroid, breast, ovarian, uterus, cervical, prostate, esophagus, stomach, colon, liver, pancreas, renal, bladder, brain, and skin cancer [348]. Vaccinations of mice with HERV epitopes were shown to be safe and able to generate tumor-specific immune cells [219,339,349,350].
Furthermore, HERV-H LTR-associating proteins 1 and 2 (HHLA1 and HHLA2) on chromosomes 3q13.13 and 8q24.22 have been shown to carry immune checkpoint functions [353]. First described by Mager et al. in 1999, HHLA1 and HHLA2 are both members of the B7 family and obtain their polyadenylation signal through HERV-H LTR regions [354,355], thus revealing a control mechanism of viral origin [356]. While both proteins are part of oncogenic signaling pathways, HHLA2 has been detected in several human cancers [195]. HHLA2 was found to be overexpressed in basal breast cancer [357], triple-negative breast cancer [357], colorectal cancer [358], lung cancer [359,360,361], liver cancer [362], bladder urothelial carcinoma [363], ccRCC [364,365,366], pancreatic cancer [367,368,369], osteosarcoma [370], oral squamous cell carcinoma [371], and many other cancers [357,364] compared to adjacent normal tissue or healthy controls. Additionally, elevated HHLA2 protein levels were associated with tumor size, tumor stage, lymph node metastasis, and low relapse-free and overall survival in these cancers [357,358,359,360,361,362,363,364,365,366,367,368,369,370,371].
In summary, HERVs are involved in various homeostatic and pathogenic pathways with potential effects on cancer development and progression. The usage of HERVs themselves as therapeutic agents, as well as the HERV proteins as tumor-specific targets, are promising but must be further evaluated to exclude any undesired side effects.