The research field of glucose biosensing has shown remarkable growth and development since the first reported enzyme electrode in 1962. Extensive research on various immobilization methods and the improvement of electron transfer efficiency between the enzyme and the electrode have led to the development of various sensing platforms that have been constantly evolving with the invention of advanced nanostructures and their nano-composites. Examples of such nanomaterials or composites include gold nanoparticles, carbon nanotubes, carbon/graphene quantum dots and chitosan hydrogel composites, all of which have been exploited due to their contributions as components of a biosensor either for improving the immobilization process or for their electrocatalytic activity towards glucose. This review aims to summarize the evolution of the biosensing aspect of these glucose sensors in terms of the various generations and recent trends based on the use of applied nanostructures for glucose detection in the presence and absence of the enzyme.
- glucose sensors
- nanomaterials
- electrochemical biosensors
1. A Brief History of Glucose Biosensing
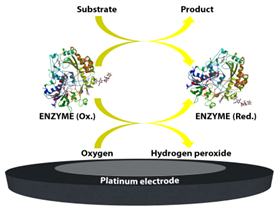
The first enzyme-based electrode for glucose detection was reported by Clark and Lyons in 1962 [1]. This device was based on glucose oxidase entrapped within a semipermeable dialysis membrane which was constructed on an oxygen electrode. Following this, Clark’s patent in 1970 demonstrated the use of enzymes to convert electroinactive substrates to electroactive substances (Figure 1). This system relied on two different electrode systems. The first electrode system, which consisted of at least one enzyme in a capillary thin layer between the electrode and the membrane, was responsible for the conversion of the substrate to electroactive material in the presence of interfering species. The second electrode system was sensitive to the interfering species available in the sample. By subtracting the measured current from the second electrode system from the measured current from the first electrode system, Clark’s patented device was successful to monitor the glucose.
Figure 1. A schematic representation of first-generation glucose biosensor.
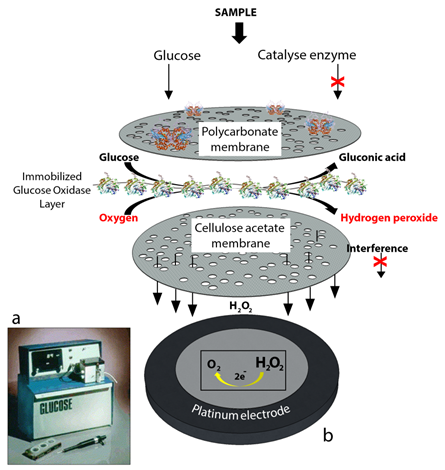
Figure 2. Second generation glucose biosensors. (a) YSI 23A glucose biosensor [2] and (b) sensor probe with immobilized enzyme membrane for the Yellow Springs Instruments (redrawn from Paul D’Orazio, Biosensors in clinical chemistry [3]).
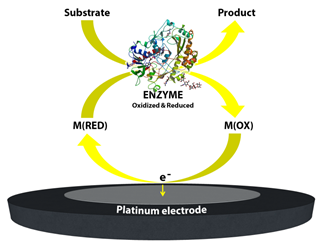
Figure 3. Schematic image of the mediated biosensor working principle, second generation biosensors.
2. Conclusions
In this entry, we have summarized the commercialization history of glucose biosensors and have described recent trends in the related research field of enzymatic, non-enzymatic, wearable non-invasive glucose biosensors as well as repurposed personal glucose meters towards non-glucose targets. Most research has proven the fact that electrochemical biosensors based on advanced nanostructures and miniaturized devices o er important sensing and diagnosis platforms demonstrating high sensitivity and selectivity towards various target analytes. Since the very first discovery of biosensors many breakthrough innovations have been demonstrated in order to improve the characteristics and performance of the desired sensing devices. First, second and third generation glucose biosensors, the use of nanomaterials or integrated polymers matrices have been described in detail for a number of specific applications. Enhanced sensitivities have been achieved for glucose biosensors by applying the use of emerging nanostructures, hybrid materials and micro-or nano technologies.
These include carbon-based nanomaterials such as graphene or graphene derivatives, carbon quantum dots, graphene quantum dots, carbon nanotubes, gold nanostructures, biocompatible hydrogel chitosan and their nano-bio-composites.
Recent advances in nanotechnology research have been accelerating the improvements in the development of enzyme free glucose sensors with excellent analytical performances. In particular metal or metal oxide nanostructures o er enhanced electroactive surface area and superior sensitivities as glucose sensors.
Both enzymatic and non-enzymatic sensing platforms still have challenges to be overcome in terms of limitations in regards to their biocompatibility, lifetime, and selectivity. Since many glucose oxidase-based electrochemical biosensors have been used as idealized model systems for the fabrication of diverse sensing platforms, they have opened up the possibility of their use in a new generation of (integrated) electrochemical biosensors for the detection of several other analytes. For example,
a combination of an electrochemical glucose biosensor with advanced DNA technologies on a small microfluidic device has been developed at low cost in order to detect miRNAs [4]. Conventional glucose meters require invasive methods for sample collection since such systems work with human blood or serum. The discomfort and pain arising from these invasive techniques have led researchers to focus on non-invasive sensing platforms which can aim to detect glucose from other
bodily fluids including tears, sweat or saliva. These achievements in the field of painless, non-invasive detection of glucose coupled with miniaturized systems can be revolutionary in terms of the control and management of diabetes. Furthermore, such achievements could lead to the development of the point-of-care devices for the other possible target analytes. The personal glucose meter is perhaps the greatest example of a point-of-care device. Commercially available glucose meters have been widely used due to their small size, easy operation, quantitative results and accuracy. These devices detect glucose from whole blood. However, the smart idea of repurposing the personal glucose meters provides the opportunity to create sensing platforms for non-glucose targets as point-of-care devices. Recently, many methods have been developed to
use personal glucose meters for bacteria, DNA, disease biomarkers, etc. detection in the presence of the invertase enzyme. For practical applications, various drawbacks associated with personal glucose meters should be studied in detail, including the limited linear range of those devices and the interference effects of the naturally occurring glucose in the biological samples. Certainly, there are also many other unexplored strategies. With this in mind, there is still a high demand and expectation for robust, accurate, sensitive, selective and cheap sensing devices particularly for the early-stage detection of biomarkers associated with various cancers and diseases such as Alzheimer’s, multiple sclerosis, etc. It is also noteworthy that the development of sensors for the reliable, continuous, real-time monitoring of glucose with high selectivity and speed also currently presents a massive challenge in the area of diabetes control.
This entry is adapted from the peer-reviewed paper 10.3390/s20216013
References
- Clark, L.C.; Lyons, C. Electrode Systems for Continuous Monitoring in Cardiovascular Surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45.
- Newman, J.D.; Turner, A.P.F. Home blood glucose biosensors: a commercial perspective. Biosens. Bioelectron. 2005, 20, 2435–2453.
- D'Orazio, P. Biosensors in clinical chemistry. Clin. Chim. Acta 2003, 334, 41–69.
- Bruch, R.; Baaske, J.; Chatelle, C.; Meirich, M.; Madlener, S.; Weber, W.; Dincer, C.; Urban, G.A. Biosensors: CRISPR/Cas13a-Powered Electrochemical Microfluidic Biosensor for Nucleic Acid Amplification-Free miRNA Diagnostics. Adv. Mater. 2019, 31, 1905311.