Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Pharmacology & Pharmacy
Saponins are one of the broadest classes of high-molecular-weight natural compounds, consisting mainly of a non-polar moiety with 27 to 30 carbons and a polar moiety containing sugars attached to the sapogenin structure. Saponins are found in more than 100 plant families as well as found in marine organisms. Saponins have several therapeutic effects, including their administration in the treatment of various cancers. These compounds also reveal noteworthy anti-angiogenesis effects as one of the critical strategies for inhibiting cancer growth and metastasis.
- triterpenoid saponins
- steroidal saponins
- angiogenesis
- VEGF
- HIF-1α
- inflammation
- signaling pathways
1. Introduction
Saponins are one of the most widespread secondary metabolites presents in the plant kingdom and marine organisms, generating stable foam in aqueous solutions, such as soap [1]. Humans have long used saponin-rich plants in their diets to maintain health and therapeutic benefits, including some Fabaceae plants [2], such as pea (Pisum sativum), beans (Phaseolus spp.), fava bean (Vicia faba), soybean (Glycine max), mung bean (Vigna radiate), and licorice (Glycyrrhiza glabra). Additionally, Allium genus plants [3] are also rich in saponins, such as garlic (Allium sativum), onion (Allium cepa), and chives (Allium schoenoprasum). Moreover, herbs, including tea (Camellia sinensis), banana (Musa spp.), and ginseng (Panax ginseng), are found as saponin sources [4]. Saponins are composed of two parts, including aglycone (sapogenin) as non-polar moiety and glycoside (saccharide chain) as polar moiety. Triterpenoid saponins and steroidal saponins with, respectively, 30 and 27 carbon atoms, are the 2 main chemical structures of sapogenin core of saponins isolated from natural sources [5]. Furthermore, farnesyl diphosphate synthesized from the mevalonate biosynthetic pathway is the main precursor of the sapogenin [6,7]. Saponins have a high structural diversity due to the presence of functional groups on the sapogenin core, the number and type of sugars in the structure, and the way sugars bind to each other and to the aglycone part. Such structural diversity causes various biological and pharmacological effects [8]. Accordingly, several effects have been reported for saponins, such as anti-microbial, anti-leishmaniasis, anti-oxidant, anti-cholesterol, anti-lipidemia, analgesic, anti-inflammation, anti-diabetic, and anti-coagulant effects. [1,9]. Saponins also revealed the prominent anti-cancer and cytotoxic activities via regulating multiple signal pathways of cellular apoptosis, cell cycle arrest, proliferation, autophagy, and inflammation [10].
Angiogenesis, as the formation of new blood vessels, is one of the most important strategies to provide the energy needed for the survival and proliferation of cancer cells. Today, the inhibition of angiogenesis is used as a new and practical idea to control the growth and metastasis of tumors [11]. Since the discovery of the significant role of angiogenesis in the growth and progression of tumors by Folkman in 1971 [12], several drugs with anti-angiogenic effects have been introduced to the global pharmaceutical market, including bevacizumab, lenalidomide, axitinib, sorafenib, and everolimus. These drugs treat cancer by inhibiting the angiogenesis triggers, including the vascular endothelial growth factor (VEGF) and hypoxia-inducible factor 1-α (HIF-1α) release [13]. On the other hand, plants and their secondary metabolites have always been considered by researchers to treat cancer. According to investigations, plant secondary metabolites have also had a significant effect on inhibiting the growth and progression of tumors through blocking angiogenesis stimulation cellular signaling pathways [14]. Vinca alkaloids and paclitaxel, which have been isolated from Catharanthus roseus (Apocynaceae) and Taxus brevifolia (Taxaceae), respectively, are among anti-angiogenesis natural products found in pharmaceutical markets [15].
Sobolewska et al. reviewed the cytotoxic effects and anti-cancer mechanisms of saponins emphasizing the structure-activity relationship and their natural sources [16,17]. In this present review, the dramatic effects of saponins have been described on inhibiting the growth and metastasis of cancer via inhibiting the gene expression of angiogenesis mediators, including VEGF/VEGFR2, HIF-1α, fibroblast growth factor 2 (FGF2), and phosphoinositide 3-kinases/protein kinase B (PI3K/Akt). The anti-cancer effects of saponin was also shown to be applied through blocking inflammatory cellular signaling pathways, including pro-inflammatory cytokines, mitogen-activated protein kinase (MAPKs) pathways, and nuclear factor κ light chain enhancer of activated B cells (NF-κB).
2. Angiogenesis: Cellular Signaling Pathways
The angiogenic process is a complex and extremely regulated action that enhances tumor progression and survival. Several signaling pathways and interconnected mediators are involved in the pathogenesis of angiogenesis that could be controlled through modulating multiple autophagy, oxidative stress, inflammatory, apoptotic pathways, and proangiogenic/antiangiogenic markers [18]. Pro-angiogenic mediators could be released through different kinds of cells, including endothelial cells (ECs), smooth muscle cells, fibroblasts, immune cells, platelets, and tumor cells [18,19]. The pro-angiogenic-signaling molecule VEGF and its cognate receptor, tyrosine kinase VEGF receptor 2 (VEGFR2), play a vital role in angiogenesis [20,21]. Epidermal growth factor (EGF), basic fibroblast growth factor (bFGF), platelet-derived growth factor (PDGF), transforming growth factor-β (TGF-β), angiopoietins, ephrins, apelin (APLN), granulocyte and granulocyte/macrophage colony-stimulating factors (G-CSF and GM-CSF), hepatocyte growth factor/scatter factor (HGF/SF), and inflammatory factors, including cytokines, such as interleukin-6 (IL-6), IL-1β, tumor necrosis factor-α (TNF-α), and chemokines are among other pro-angiogenic agents [19,21,22,23,24,25]. Inflammation is one of the main lethal factors in angiogenesis and cancer, which cause DNA damages through several macrophage migration inhibitory factor (MIF) and reactive oxygen species (ROS) and reactive nitrogen species (RNS) production [26]. These mediators contribute to the angiogenesis through the upregulation of transcription factors, such as MAPKs, NF-κB, signal transducer and activator of transcription 3 (STAT3), and mammalian target of rapamycin (mTOR). Recent studies demonstrated MAPK-signaling cascade and its main subfamilies, such as extracellular signal-regulated kinase (ERK), c-Jun NH2-terminal kinase (JNK), and p38 plays pivotal roles in inflammation/oxidative stress-associated angiogenesis. Besides phosphatases and tensin homolog (PTEN)/PI3K/Akt/mTOR, Janus kinase (JAK)/STAT and Ras/Raf/mitogen-activated protein kinase (MEK)/ERK/MAPK signaling pathways are also interconnected with the modulation of inflammation, oxidative stress, and cancer cells survival [27,28,29,30,31,32,33]. Oxidative stress is another trigger of angiogenesis that linked with aforementioned signaling pathways. Oxidative stress is caused by an imbalance between ROS/RNS production and antioxidants factors, including catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx), and glutathione (GSH) [34]. Angiogenic inhibitors, including angioarrestin, angiostatin, endostatin, tumstatin, fibronectin fragment, IL-1, IL-12, interferons, canstatin, retinoic acid, arrestin, tissue inhibitors of metalloproteinases (TIMPs), multimerin 2, and angiogenic activators act together to maintain the balance of angiogenesis [13,19,23,35,36,37]. Angiogenesis is accomplished when stimulating factors, such as VEGF, angiopoietin-1 (ANG-1), and placental growth factor (PLGF), permeabilize the vessels. These factors also involved in the formation of new vessels. In addition, angiopoietin, angiogenin, TGF α/β, and matrix metalloproteinases (MMPs) are among other angiogenic stimulators [35,38,39]. Moreover, recent studies showed microRNAs (miRNAs) play a significant role in metabolism, apoptosis, protein secretion, cell proliferation, division, and differentiation that could modulate different stages of angiogenesis by targeting numerous genes within various signaling pathways. Thus, they could be new therapeutic targets in regulating angiogenesis [40]. In addition, hypoxia disrupts the balance between pro-angiogenic and antiangiogenic factors leading to the upregulation of HIF-1α, which suppresses antiangiogenic factors expression while promoting the pro-angiogenesis factors expression [19,41]. HIF-1α is the main stimulus for elevated VEGF, FGF, and PDGF production in cancer [23]. Hence, all the markers in the aforementioned signaling pathways could be considered effective therapeutic targets in angiogenesis.
3. Targeting Tumor Cells with Anti-Angiogenic Agents: Recent Advances
To stop angiogenesis, it is necessary to use anti-angiogenic factors or agents that decrease the production of pro-angiogenic markers, prevent them from binding to their receptors, or stop their functions [42]. Monoclonal antibodies (mAbs), small molecule inhibitors, VEGF inhibitors, tyrosine kinase inhibitors, gene therapy, and RNA interference (RNAi) therapy are auspicious anti-angiogenic interventions [19,43,44]. VEGF/VEGFR and interconnected downstream pathways have been the crucial targets of anti-angiogenic drugs. Small molecules inhibitors and mAbs modulating cell growth, differentiation, and angiogenesis through targeting VEGFRs, FGF receptors (FGFRs), EGFR receptors (EGFRs), PDGF receptors (PDGFR-α, PDGFR-β), Fms-like tyrosine kinase 3, and signaling proteins, including Raf, MAPK, mTOR, and PI3K [19,42,43,44]. Bevacizumab, aflibercept, and ramucirumab are monoclonal antibodies that exert anti-angiogenic effects by targeting the VEGF/VEGFR signaling pathway [21,43,44,45]. Receptor tyrosine kinase inhibitor small molecules (RTKIs) suppress signals inside blood vessels cells and are active against a numerous range of receptors involved in angiogenesis, including VEGFR, PDGFR, FGFR, rearranged during transfection (RET), and Tie receptors [43]. Sorafenib and sunitinib are among RTKIs that block angiogenesis and tumor growth via targeting the RAF/MEK/ERK signaling pathway, VEGFR-2, PDGFRB, and colony-stimulating factor-1 (CSF-1) [43,44]. The angiopoietins (Ang1–4)–Tie-axis is another pathway in angiogenesis. Ang1 and Ang2 expressions increased in many tumors. Ang1 binds to the Tie2 receptor and leads to a decline in vascular permeability and raised vessel stabilization, while Ang2 induces neovascularization and ECs migration and proliferation in response to pro-angiogenic markers [44,46,47]. Hence, a higher ratio of Ang1 to Ang2 predicts better outcomes. Trebananib showed promising antiangiogenics effects by targeting the Ang/Tie-axis in phase II trials [48]. Recent studies showed phytochemicals, such as alkaloids, saponins, tanshinone, coumarins, flavonoids and artemisinin, allow for the activity of modulating angiogenesis through regulating pro-angiogenic and anti-angiogenic factors [49,50]. In addition, vinca alkaloids (e.g., vinblastine, vincristine, vinorelbine, and vindesine), podophyllotoxin and its derivations (e.g., irinothecan, topothecan), taxanes (e.g., docetaxel, paclitaxel), camptothecins, and anthracyclines (e.g., daunorubicin, idarubicin, doxorubicin, and epirubicin) are effective herbal medicines on angiogenesis/cancer [19,24,49,50,51]. In recent decades, natural products have attracted great interest in combating tumor angiogenesis due to their fewer side effects and multi-functional mechanisms of action. Therefore, natural products and phytochemicals could be promising sources for the prevention or/and treatment of cancer.
4. Saponins
4.1. Chemistry, Biosynthesis, and Natural Sources
Saponins are a large group of secondary metabolites that are abundant in natural sources, including plants and marine organisms. These compounds have a high molecular weight, containing 27–30 carbons (C). In their structure, C3 is bound to the hydroxyl group in all aglycone saponins [52]. Saponins possess a polar/non-polar structure. In their polar sections, saponins have sugar chains (glycosylated forms), while the sapogenin structure makes the nonpolar part. Such chemical properties cause soap-like foams behaviors in aqueous solutions for saponins [53].
Squalene and 2,3-oxidosqualene are critical precursors in the biosynthesis of saponins. Accordingly, two units of farnesyl pyrophosphate formed in the mevalonate biosynthetic pathway and converted to squalene in the presence of the enzyme squalene synthase. The produced squalene is then converted to 2,3-oxidosqualene by the squalene epoxidase enzyme. Consequently, squalene and 2,3-oxidosqualene are converted to various aglycone saponins by different enzymes, cyclase and cytochrome P450. Finally, aglycone saponins are glycosylated by glycosyltransferase enzymes [6,7,54] (Figure 1).
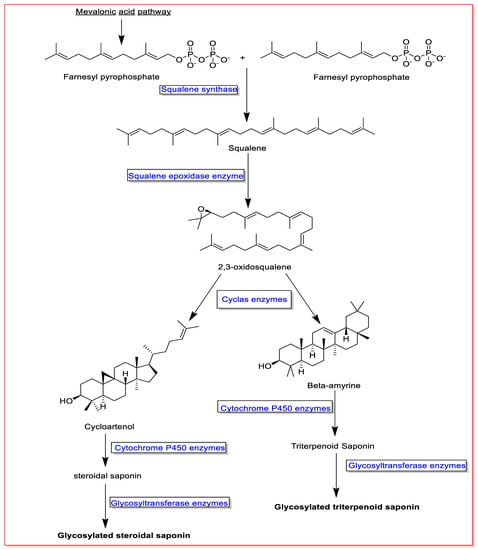
Figure 1. An overview on saponin biosynthesis pathway.
Saponins are typically divided into two main categories, including triterpenoid saponins (C30, Figure 2A) and steroidal saponins (C27, Figure 2B). However, alkaloidal saponins (Figure 2C), which contain nitrogen atoms, are also classified in this category [5,55]. Consequently, steroidal saponins are divided into three categories, including spirostanes, furostanes, and cholestanes or open-chain steroidal saponins (Figure 2B). The spirostanes have a common spiro-carbon at the junction of two heterocyclic rings of furan and pyran in their structure and A and B rings are mainly trans isomer. The A and B rings of furostanes are found in both the cis and trans isomeric position and mainly consist of four hydrocarbon rings attached to a furan ring. Consistently, cholestane steroids do not have O-heterocyclic rings, and there is an opened ring in this group of saponins [52,56].
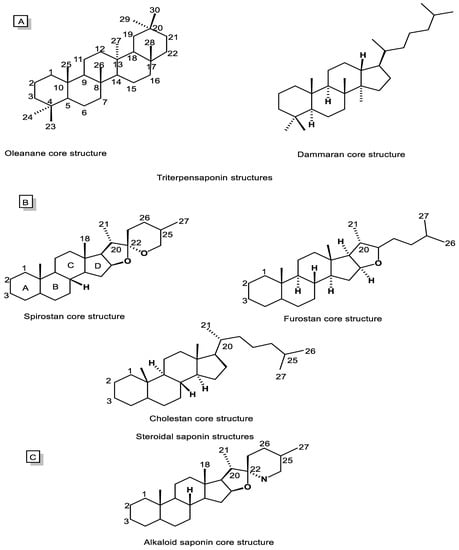
Figure 2. Chemical structures of saponin cores. (A) Triterpenoid saponins, (B) Steroidal saponins, and (C) Alkaloidal saponins.
Oleananes and dammaranes (Figure 2A), with, respectively, five and four hydrocarbon rings, are two main groups of triterpenoid saponins isolated from natural sources, especially the plant kingdom. The terpenoid saponins rings are in an all-trans isomeric position. Additionally, their aglycone mostly contains functional groups including COOH, OH, and CH2OH at C28, C3, and C24, respectively [5,53,57].
Natural sources-isolated saponins are in glycosylated form and contain various saccharide chains. These saccharide chains are mainly oligosaccharides and composed of Dextro (D) or Leavo (L) isomers of sugars, such as glucose, galactose, rhamnose, arabinose, xylose, and glucuronic acid. However, sugars, such as quinovose, apiose, and fucose, are also found at a lesser frequency [53,58,59,60,61]. According to the number of saccharide chains attached to the saponin structure, these compounds are divided into two main categories: monodsmosidic and bidesmosidic, although in limited cases, tridesmoside saponins are also seen [62]. Although steroid saponins are generally monodsmosidic and are usually attached to the sugar chain at the C3 position, terpenoid saponins are bidesmosidic. Furthermore, tridesmoside, and the sugar chains, in addition to C3, are attached to the functional groups in C28 and C24 carbon. However, in some cases, the saccharide chain may bind to other carbons in the saponins structure [63,64,65].
Saponins are widely found in more than 100 families of dicotyledonous and monocotyledonous plants. These compounds are presented in different parts of the plants, including leaves, stems, roots, flowers, bark, and fruits, accumulate and thereby play protective roles against herbivores, microorganisms, and environmental stresses such as drought stress and temperature changes [66,67]. Steroid saponins are mostly found in monocotyledonous plants, such as Dioscoreaceae, Asparagaceae, Liliaceae, and Amaryllidaceae. Dicotyledonous families, such as Fabaceae, Araliaceae, Quillajaceae, Polygalaceae, Caryophyllaceae, Primulaceae, and Theaceae, mostly contain terpenoid saponins. Alkaloidal saponins are also isolated from the Solanaceae family [56,68,69]. Several steroidal saponins, such as dioscin, gracillin, deltonin, and parrisaponin, are isolated from Dioscorea genus [68]. Patricia Y et al. isolated shatavarin V as a new spirostanol saponin from the Asparagus racemosus (Asparagaceae) root [70]. Sobolewska et al. have introduced more than 350 steroidal saponins from Allium species (Liliaceae) in their review article [52]. In another study, 10 new terpenoid saponins were separated from the root of Glycyrrhiza glabra (Fabaceae), such as 11-deoxorhaoglycyrrhizin, 30-hydroxyglycyrrhizin, and 20α-rhaoglycyrrhizin [71]. Additionally, more than 100 saponins were isolated from Quillaja saponaria bark (Quillajaceae), with quillaic acid and its derivatives among the most well-known [72]. Panax ginseng (Araliaceae) is one of the rich sources of dammarane-type saponins, including ginsenoside derivatives [73]. Due to the presence of two or three hydroxyl groups in the chemical structure of ginsenosides, they are categorized into two main groups, including protopanaxadiol (Rb1, Rb2, Rg3, Rc, Rd, and Rh2) and protopanaxatriols (ginsenoside Rg1, Rg2, Rg3, Re, and Rh) [74,75]. Furthermore, nowadays, sea organisms, such as sea cucumbers, starfish, and sponges, have attracted the attention of researchers as rich sources of saponins [76,77,78]. Bahrami et al. isolated and identified 89 types of saponins, including terpenoid saponins, Holothurinoside and Holothurin derivatives, from a species of sea cucumber (Holothuria lessoni) [79].
In summary, due to their unique chemical structure and structural diversity, saponins have shown a bright future among natural compounds.
4.2. Pharmacological and Biological Activities
The structural diversity of saponins have led to their various biological and pharmacological effects. In addition to their biological effects, such as cytotoxicity [17], antibacterial [80,81], anti-viral [82], anti-fungal [83,84], anti-leishmania [85,86], anti-inflammation [87,88], and anti-oxidant [89,90] mechanisms, saponins have shown prominent pharmacological effects, such as cardioprotective [91], neuroprotective [92], anti-cancer [93,94], hepatoprotective [95], wound healing [96,97], analgesic [98,99], anti-rheumatoid [100,101] anti-convulsant [102] and immunomodulatory [103,104] activities. More recently, saponins have also been used as adjuvants in vaccines [105,106]. QS-21, isolated from Quillaja saponaria bark (Quillajaceae), is one of the saponins that has passed clinical trials in different phases for use as an adjuvant in vaccination [107]. Accordingly, studies showed the beneficial therapeutic effects of saponins on various diseases, including metabolic diseases, such as obesity [108,109], diabetes [110,111], hypercholesterolemia [112], hypertension [113,114], and osteoporosis [115].
Dutta et al. showed that rasmuside A, as a steroidal saponin isolated from Asparagus racemosus fruits, had an anti-leishmaniasis effect on the Leishmania donovani strain at 1.31 ug/mL [116]. The saponin-rich fraction of green tea seeds (Camellia sinensis, Theaceae) showed significant anti-bacterial effects against Escherichia coli, Staphylococcus aureus and Salmonella spp. via the penetration and destruction of the bacterial cell membrane/wall [117]. Another study showed anti-oxidant and anti-inflammation activities for Camellia sinensis root saponins [118]. Aginoside, as a spirostane saponin isolated from Allium nigrum (Liliaceae) bulbs, showed strong anti-fungal activities at 400 ppm against wild fungi, such as Fusarium spp., that invade plants [119]. Moreover, Li et al. showed that the saponin-rich extract of Panax ginseng steamed root ameliorated the ischemic injuries at 200 mg/kg and 400 mg/kg, orally via modifying the hemodynamic parameters, such as the left ventricular systolic pressure and heart rate, as well as a decreasing of the intracellular calcium ion overload [120]. Additionally, a clinical study has been designed on the healing effects of Panax notoginseng saponins on patients with hypertensive intracerebral hemorrhage (NCT02999048). Onjisaponin derivatives, as the triterpenoid saponins isolated from Polygala tenuifolia root (Polygalaceae), showed prominent neuroprotective effects and increased the survival of PC21 cell lines against glutamate-induced neurotoxicity [121]. Additionally, Meng et al. showed in vitro (0.5, 1, and 2.5 μM) and in vivo (2.5 mg/kg, 5 mg/kg, and 10 mg/kg, intragastrically, rats) potentials of Paris saponin VII as a steroidal saponin separated and purified from the rhizome of Trillium tschonoskii Maxim (Melanthiaceae). In their study, Paris saponin VII ameliorated rheumatoid arthritis by inhibiting the expression of inflammatory signaling pathways, such as mitogen-activated protein kinase, and reducing inflammatory cytokines [100]. On the other hand, the saponin-rich extract of sea cucumber (Pearsonothuria graeff) revealed marked anti-obesity and anti-hyperlipidemia effects in the rat fed with 0.08% saponin-rich extract and a high-fat diet for eight weeks [122]. Macrophyllosaponin B and astragaloside VII, as two terpenoid saponins found in Astragalus spp., showed regulatory effects on the immune system at 60 μg/albino mice intraperitoneal (i.p.) via blocking the activities of interlukin-4 and stimulating the expression of interlukin-2 and interferon-γ [123]. The saponin-rich fraction of Clerodendrum infortunatum leaves (Lamiaceae) showed anti-convulsant effects at 45 mg/kg, i.p., and relieved the central and peripheral pain at 35 and 40 mg/kg, i.p., respectively, in mice [124]. In addition, several clinical trials have been conducted on the beneficial therapeutic effects of saponins and plants rich in saponins, such as onion (Allium cepa, Araliaceae) and fenugreek (Trigonella foenum-graecum, Fabaceae) [110,125,126,127,128]. So far, several clinical trials have been reported on the therapeutic effects of ginsenoside saponins on various diseases, including cardiovascular disorders, diabetes, blood lipids, high blood pressure, and obesity [129,130].
Furthermore, saponins have shown significant cytotoxic and anti-cancer activity through various mechanisms in in vivo and in vitro studies, which we will discuss in more detail in the next section. Overall, saponins have high therapeutic potential and could be introduced as promising candidates for the treatment of complex diseases, such as metabolic disorders and cancer.
4.3. An Overview on the Anti-Cancer Mechanisms of Saponins
According to the World Health Organization, cancer is rapidly spreading worldwide and has been the main cause of death in more than 60% of countries (112 out of 183 countries studied) in the world [131]. Therefore, researchers in the field of cancer are trying to find effective treatments for it. Due to their low side effects, multi-therapeutic targeting, and in most cases, their low-cost phytochemicals have been promising candidates for cancer treatment. Among the phytochemicals, saponins have been prominent anti-cancer candidates in vitro and in vitro [10,94].
4.4. Anti-Angiogenic Potentials
The inhibition of angiogenesis and direct killing of cancer cells are two main strategies in the treatment of cancer [172]. Ever since Folkman proposed the theory of angiogenesis inhibition for the treatment of cancer in 1971, many efforts have been made to find molecules with anti-angiogenic effects [12]. These investigations led to the introduction of some drugs, such as sorafenib, bevacizumab, sunitinib, everolimus, and axitinib, with anti-angiogenic effects in the treatment of various cancers [13]. Natural sources, especially phytochemicals, have been among the main candidates for the discovery of molecules with anti-angiogenic effects [173]. Alkaloid-based drugs (e.g., vincristine, vinblastine, and camptothecin) [174], terpenoid-based drugs (e.g., paclitaxel) [175], flavonoid-based drugs (e.g., naringenin, kaempferol, and hesperidin) [176], and coumarin-based drugs (e.g., galbanic acid, esculetin, and daphnetin) [19] are among the major phytochemicals reported to inhibit angiogenesis. Additionally, Saponins, as one of the largest groups of natural compounds, have shown notable anti-angiogenic effects in the treatment of various cancers, in vitro and in vivo.
This entry is adapted from the peer-reviewed paper 10.3390/metabo13030323
This entry is offline, you can click here to edit this entry!
