Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Medicine, Research & Experimental
Adipokines secreted from white adipose tissue (WAT) and bone marrow adipose tissue (BMAT) exerts endocrine and paracrine effects on the survival and function of osteoblasts and osteoclasts.
- adipokines
- bone metabolism
- osteoblast
- osteoclast
- bone turnover markers
- bone mineral density
- osteoporosis
1. Introduction
Adipose tissue secreted adipokines have both pro-inflammatory (leptin, resistin, RBP-4, lipocalin 2, NAMPT) and anti-inflammatory properties (omentin-1, adiponectin, SFRP5) that mediate pathophysiology of several bone diseases [1]. The more recent adipokines such as omentin-1 and vaspin not only have insulin-sensitizing properties and protective effects on cardiovascular health [2,3], but also have a significant role in bone metabolism. A few studies have found a positive association between vaspin and BMD serving as a protective biomarker against osteoporosis [3,4], while the effects of omentin-1 on BMD are conflicting [5,6]. The correlation between chemerin with BMD has been consistently negative [7,8,9], whereas no convincing evidence has been found on the association between visfatin [10,11] and lipocalin 2 [12,13] with BMD. A few other adipokines, such as RBP-4, have been shown to promote osteogenesis in vivo only, while adipokine apelin enhances human osteoblast proliferation both in vitro and in vivo [14,15,16]. In animals, nesfatin-1 may have a potential protective role on bone metabolism, however, evidence of a similar effect in humans is lacking [17,18].
2. Adipokines
2.1. Adiponectin
Adiponectin is one of the predominant adipokines that plays a crucial role in obesity, glucose, and lipid metabolism, as well as cardiovascular disease [19]. The role of adiponectin signaling in bone homeostasis remains complex, in part due to its multiple isoforms and receptor sub-types and conflicting results from animal versus human studies.
2.2. Leptin
Leptin is yet another adipokine that is involved in energy homeostasis and bone metabolism. Evidence from previous studies have demonstrated that leptin regulates bone mass by both peripheral and central mechanisms, although in vivo effects remain controversial to date [30]. Herein we summarize our current knowledge and understanding of leptin’s effect on bone metabolism.
2.3. Visfatin
Visfatin, also known as pre-B cell colony-enhancing factor (PBEF) or nicotinamide phosphoribosyltransferase (NAMPT), is primarily synthesized by visceral adipose tissue [50]. NAMPT/visfatin is involved in energy metabolism and is also known to have pro-inflammatory properties [51] and, thus, may be helpful as a potential biomarker for obesity, insulin resistance, and type 2 diabetes (T2D) status [52].
2.4. Chemerin
Chemerin, also known as retinoic acid receptor responder 2 (Rarres2), or tazarotene-induced gene 2 (TIG2), was first described by Nagpal et al. in 1997 and was originally identified as a gene upregulated in psoriatic skin by synthetic retinoid [58]. Further studies revealed that chemerin is highly expressed in white adipose tissue (WAT), liver, and lung, while its receptor chemokine-like receptor 1 (CMKLR1) is predominantly expressed in adipocyte and immune cells [59,60]
In 2007, Bozaoglu et al. reported the level of chemerin and its receptor, chemokine-like receptor 1 (CMKLR1), were significantly high in individuals with obesity and T2D [59]. Several other studies have shown an association between elevation in chemerin expression and metabolic and inflammatory diseases including psoriasis, obesity, T2D metabolic syndrome, and cardiovascular disease [59,61]. Moreover, the dysregulated ratio of chemerin to adiponectin could play a role in metabolic syndrome [62]. However, its role as a regulator of bone mass needs further investigation.
2.5. Omentin-1
Omentin-1, also known as intelectin-1, was originally found in Paneth cells of the small intestine and is an intestinal lactoferrin receptor [67]. It is one of the novel adipocytokines that influences glucose metabolism, serving as a predictor for diabetes [68], and has anti-inflammatory [69], anti-atherosclerotic, and cardiovascular protective effects [70]. Some experimental evidence indicates that omentin-1 has anti-inflammatory, antioxidative, anti-apoptotic, and microbial defense roles [71].
2.6. Lipocalin-2 (LCN2)
Lipocalin-2 (LCN2) is a novel osteoblast-derived adipokine consisting of 198 amino acids. It is also known as Neutrophil gelatinase-associated lipocalin (NGAL) [80]. Neilsen et al.1996 proposed that it might be a scavenger of bacterial products at sites of inflammation [81]. Recent advances in knowledge suggests that secretion of LCN2 is 10-fold higher in osteoblasts compared to white adipose tissue (WAT) as was previously understood [82]. Experiments in mice demonstrate that osteoblast-derived LCN2 maintains glucose homeostasis by inducing insulin secretion and improves glucose tolerance and insulin sensitivity. In addition, it regulates appetite suppression in a MC4R-dependent manner [82]. While the role of LCN2 in regulating gluco-metabolic parameters and the effect of metabolic dysregulation on LCN2 remain complex [83,84,85] evidence for its regulatory effects on bone metabolism is rapidly emerging.
2.7. Resistin
Resistin is an adipocyte-derived cytokine that has been linked to obesity, diabetes, insulin resistance, and atherosclerosis [92,93,94,95]. Resistin is also known to regulate the production of pro-inflammatory cytokines [96] and plays a role in energy homeostasis [97].
2.8. Nesfatin-1
In 2006, Oh-1 and colleagues identified a novel anorexigenic adipokine nesfatin, also known as non-esterified fatty acid/nucleobindin2 (NEFA/NUCB2) produced in the hypothalamus and released as a fragment, nesfatin-1 into the circulation. Nesfatin-1 is located in the N terminus of NUCB2 and is indispensable to the induction of satiety in vivo [108]. Studies have shown high serum levels of nesfatin-1 in patients with OA [109]. In vitro nesfatin-1 induces the production of pro-inflammatory agents, such as COX-2, IL-8, IL-6, and MIP-1α in human primary chondrocytes from OA patients [110]. While animal studies have shown the anti-inflammatory and anti-apoptotic effects of nesfatin-1 on rat chondrocytes and potential effects on ameliorating OA [111], currently there is limited understanding of the role of nesfatin-1 on bone metabolism [112].
2.9. RBP-4
RBP-4 is an adipocyte-secreted molecule that is elevated in the serum before the development of frank diabetes [113]. RBP-4 is correlated with the magnitude of insulin resistance independent of obesity [113], however, the effect of RBP-4 on bone metabolism is poorly understood.
2.10. Apelin
Apelin is a recently discovered peptide that is the endogenous ligand for the orphan G-protein-coupled receptor APJ. Apelin and APJ are expressed in a variety of tissues with predominant protective effects on cardiac vasculature [119]. Animal studies have revealed exciting development in the treatment of obesity and diabetes using apelin analogs [120]. Apelin has also been implicated in the regulation of skeletal homeostasis as well as in the pathogenesis of osteoarthritis (OA).
2.11. Vaspin
Vaspin is a novel adipokine derived from visceral adipose tissue identified as a member of the serine protease inhibitor family that exerts insulin-sensitizing effects in obesity [2]. Vaspin has protective effects on endothelial progenitors and anti-atherogenic properties [3]. Evidence suggests that it could potentially ameliorate vascular complications from hyperglycemia in patients with T2D [3]. However, knowledge of its role in regulating bone metabolism is limited but rapidly emerging.
3. Cross Talk between Adipokines and Bone
3.1. Transcriptional Factors That Modulate Adipocytes and Subsequent Effect on Osteoblastogenesis
The fate of differentiation of bone marrow MSCs, to either adipogenic or osteoblastogenic programs, plays an important role in the regulation of bone homeostasis [136]. Studies confirmed that extracellular signaling proteins, including the adipocyte-specific peroxisome proliferator-activated receptor gamma (PPARγ) or myogenic osteoblast initiation (inhibition of PPARγ or activation of Runx2/osterix) transcription factors, can alter the differentiation of osteoblasts [137,138,139]. Apart from Wnt1 signaling, several BMPs also stimulate osteogenesis [140]. For example, activation of type1A BMP receptor (BMPR-1A) induces PPARγ expression and subsequent adipocyte differentiation [141]. On the contrary, activation of the type IB BMP receptor (BMPR-IB) induces Runx2 expression and favors osteoblastogenesis [142]. IGF1 along with insulin was reported to activate the signaling cascade of differentiation of MSCs to adipogenic or osteoblastogenic pathways in patients with obesity and diabetes [143]. The crucial shift of MSC differentiation to adipogenic rather than osteogenic lineage is linked to reduced bone formation observed in both obese and diabetic patients. Of all factors, PPARγ remains the key driver of adipocyte differentiation by binding to the c-fos promoter of hematopoietic stem cells (HSCs), thereby abrogating myogenic and stimulating osteoclastogenic cascade. Consistent with these findings, selective deletion of osteoclastogenic PPARγ has been found to increase bone volume from reduced bone resorption [144]. In addition, the collagen composition of the bone matrix is also believed to have an influence on MSC differentiation to myogenic lineage. This was confirmed by a study of cultured MSCs on denatured collagen which resulted in the promotion of adipogenesis [145]. Thus, as illustrated in Figure 1, a shift in MSC differentiation to adipogenesis may be linked to PPARγ and other transcriptional factors leading to excessive or abnormal bone resorption. The cross-talk between bone forming osteoblasts and osteoclasts, can be decoupled by number of pro-adipogenic factors leading to disruption of normal homeostasis of bone. This tight regulation might be lost/enhanced by the several adipokine derivatives that can activate osteoclastogenesis in PPARγ-dependent manner.
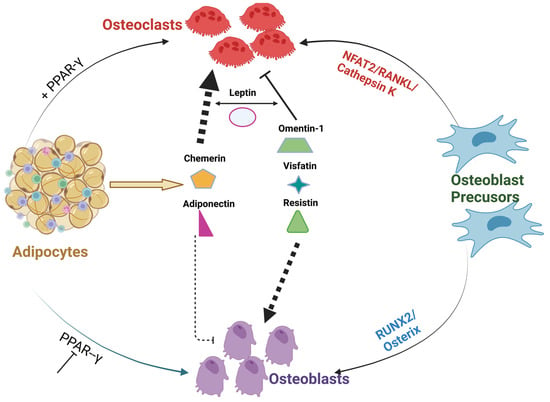
Figure 1. Cross-talk between different Adipocyte derived factors and bone. The above figure illustrates PPARγ inhibition or activation along with adipocyte-derived adipokines can alter or disrupt the communication between osteoblasts and osteoclasts. Adipokines; omentin visfatin and resistin enhance osteoblast differentiation and inhibit osteoclast formation. On the contrary, adipokines; leptin, chemerin, and adiponectin abrogate osteoblast formation and, thus, resulting in alterations in the homeostasis of bone [142,143,144].
3.2. Role of Adipokine Machinery on Osteoblast Differentiation and Bone Resorption
Apart from adipocyte differentiation, in the presence of PPARγ, the release of signaling proteins NFAT2/RANKL/Cathepsin can also affect bone homeostasis [146]. Adiponectin, the primary adipokine, has been reported to have a direct negative effect on MSC osteoblastogenesis [147]. Recently, this finding was supported by a study in aging and postmenopausal women have confirmed an inverse relationship between serum adiponectin levels and BMD [148]. On the contrary, the mode of action of Leptin remains unclear, whereas, in some studies, leptin acts directly on precursor cells to promote osteoblast differentiation and proliferation but in some studies, leptin was linked to reduced bone mass [31,149,150]. Chemerin, a novel adipokine with its receptor CMKLR1, has direct interaction with PPARc and promotes MSC adipogenesis [151]. In some other studies, simultaneous knock-out studies of CMKLR1, increased osteoblastogenesis by upregulation of osterix and suppressed osteoclast differentiation [63,79,152], but overall, the role of chemerin in relation to bone homeostasis remains unclear.
Similarly, as demonstrated in Figure 2, vaspin interacts with Grp78 a glucose-regulated protein, to induce intracellular signaling (PI3K/AMPK pathway) in vascular smooth muscle cells that not only improves glucose tolerance and insulin sensitivity but also enhance osteoblastogenesis in rats. [153]. Novel adipokines of apelin with receptor APJ [154], (angiotensin II receptor like-1), vaspin, and nesfatin-1, when bound to G-protein, coupled receptors (GPCRs) and cannabinoid receptors (CR), are known to maintain glucose tolerance [155,156] Additionally, in some studies of obesity, vaspin levels are low along with suppression of leptin and TNF-α synthesis [157].
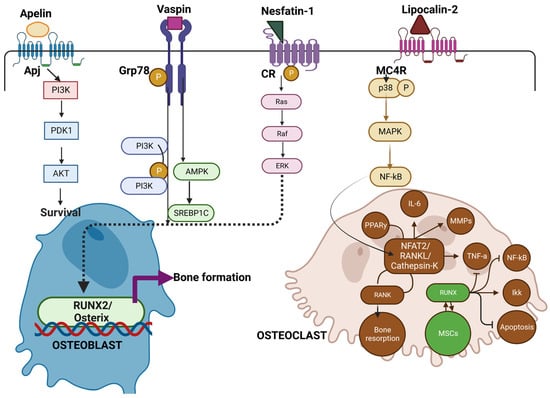
Figure 2. Illustrates the role of Novel adipokines role in bone metabolism. Apelin, vaspin, and Nesfatin when bound to receptors of APJ, Grp78, and Cannabinoid receptors (CR) activate Pi3k -Akt pathway and Ras pathway for subsequent osteoblast survival and RUNX2/Osterix pathway leading to bone formation. On the other hand, lipocalin (LCN2) binds to the MC4R receptor complex on OCPs, inducing the p38 MAP kinase pathway, then binds to Sp1 sites on the RANK promoter, activating RANK expression. This in turn favors osteoclast formation and bone resorption. Thus, novel adipokines mediating the crosstalk between osteoblast and osteoclast add on a new mechanism by which Osteoblast can control bone resorption [147,148,149,150].
However, like other adipokines, the overall functional aspect of vaspin is unknown. On the other hand, LCN2 is a glycoprotein binding to the MC4R receptor and is involved in iron homeostasis. LCN2 is abundantly expressed in WAT and is induced by inflammatory stimuli by activation of NF-Kb [158]. Serum concentrations of this protein are positively associated with adiposity, insulin resistance hyperglycemia, insulin resistance, and CRP levels [159]. LCN2 binds to MC4R to initiate the p38-MAPK pathway and results in the activation of osteoclast precursors [160]. Even though most of the adipokines initiate osteoblast activation and subsequent bone formation, the perspective and role of various novel adipokines on bone metabolism need to be re-examined to better understand the pathophysiology of bone.
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines11020644
This entry is offline, you can click here to edit this entry!
