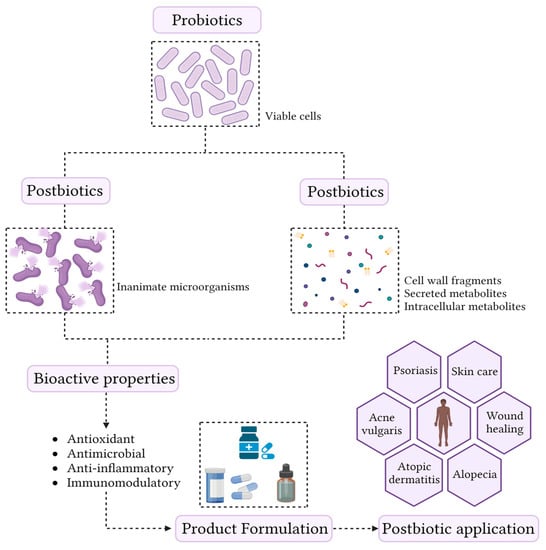
1. Overview of Postbiotics
Although the beneficial health effects promoted by probiotics are often associated with cell viability, current knowledge allows us to state that not all mechanisms are directly related to this characteristic [
49]. Recently, it has been shown that non-viable microorganisms (postbiotics) and the by-product of microbial metabolism can also confer anti-inflammatory, antimicrobial, antioxidant, and immunomodulatory effects on the host [
7]. Therefore, postbiotics represent new groups of compounds that exhibit biological activities [
50], and are shown in (
Figure 1).
Figure 1. Conceptualization of the term postbiotic and schematic representation of the skin health benefits.
The most important and well-known postbiotics are bacteriocins, short-chain fatty acids (SCFA), organic acids, and tryptophan (Trp) [
50,
51]. The mechanisms of action of these compounds are similar to those of probiotics and can be classified as direct or indirect. The direct mechanisms result in an action on the host cell, while the indirect action can stimulate the growth of microorganisms beneficial to health and inhibit the growth of pathogenic and opportunistic microorganisms [
51,
52]. However, these effects have been described primarily in the gut, but the impact of these compounds on epithelial cells and the skin microbiome have not yet been fully elucidated.
From a technological point of view, postbiotics have several advantages over probiotics, which include longer shelf life, stability over a wide temperature and pH range, defined chemical composition, no ability to transfer antibiotic resistances, and the fact that they can be used in immunosuppressed people [
51]. In addition, postbiotics eliminate the need to maintain viable cells. These characteristics allow postbiotics to be incorporated into formulations, which makes their application innovative in the cosmetic ingredient market. Although this is a relatively new area, postbiotics are an emerging trend. Currently, there are about 17 companies that commercialize probiotics for cosmetic purposes, and most of these compounds are derived from bacteria belonging to the
Lactobacillus genus. Among eukaryotes, these products are obtained almost exclusively from the yeast
Saccharomyces cerevisiae [
7]. There is a great prospect for the growth and development of the postbiotics market. However, this new area of science still lacks research, and new efforts should be made to identify and characterize new postbiotics from different microbial strains, whether probiotic or not.
2. Postbiotic Production Process
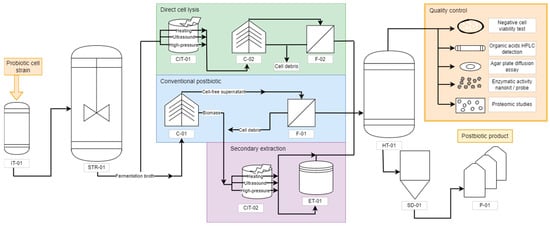
Using the information available in the literature, a flowsheet to produce postbiotics has been proposed (
Figure 2). It is highlighted to show the main differences in the downstream steps to assist researchers and companies in decision measures. Initially, the proper probiotic strain is inserted in an inoculum tank bioreactor (IT-01) with small-scale volumes (1 to 10 L). After initial growth and establishing the exponential phase, the probiotic cells are transferred to a larger scale bioreactor (over 10 L), usually a stirring tank reactor operating in batches without aeration, as both
Lactobacillus and
Saccharomyces are favored by anaerobic conditions [
53]. After reaching adequate biomass concentration, the fermentation broth is removed from the bioreactor and can be destined to three main postbiotic downstream processes: (i) conventional biomass removal; (ii) direct cell lysis and subsequent cell debris removal; and (iii) probiotic-derived secondary metabolite extraction. The conventional postbiotic production pathway begins with a centrifuge (C-01) that separates the biomass from the cell-free supernatant. The supernatant undergoes a filtration (F-01) with a rotating drum filter, and it is directed to a homogenizing tank (HT-01).
Figure 2. Flowchart of postbiotic production. IT = inoculum tank; STR = stirred tank reactor; C = centrifuge; F = filter; CIT = cell inactivation tank; ET = extraction tank; HT = homogenizing tank; SD = spray dryer; P = packaging.
The fermentation broth can also be directly destined to cell lysis without previous concentration. The inactivation methods include ultrasound, high-pressure breakage, and enzymatic treatment, performed in the cell inactivation tank (CIT-01) [
54,
55]. This will liberate both intracellular and cell membrane/wall molecules into the solution, requiring further centrifugation (C-02) and filtration (F-02) to remove large cell debris before being directed to a homogenizing tank (HT-01). Another possibility is to perform cell lysis of the centrifuged biomass in a secondary pathway. The resulting biomass of the C-01 centrifuge can be coupled with the cell debris of other unit operations, such as the filtration F-01, and inactivated, resulting in other postbiotic product. The same inactivation methods can be applied to the C-01 biomass in a cell inactivation tank (CIT-02). Whenever necessary, the inactivation can be followed by specific extraction methods in an extraction tank (ET-01), where solvents can remove specific molecules from the original solution due to their stronger solubilizing properties—for instance, using ethanol or ethyl acetate to extract carbenicillin, cephalexin, cephalothin, and tetracycline [
56]. After the inactivation, with or without extraction, the metabolites are destined to a homogenizing tank (HT-01).
The homogenizing tank is positioned at the end of each strategy to guarantee that the product will present a similar composition in all fractions. The most promising alternative when the desired product is in a solid particulate form is the spray dryer (SD-01) followed by packaging (P-01). Although the spray dryer uses heated air, it is less aggressive than the inactivation methods and generally presents protective compounds that are added before drying to soluble starch, such as glucose, sucrose, lactose, and maltodextrin [
57].
Before being released into the market, the postbiotic should undergo quality control for its composition. Beyond the traditional beneficial features characterization, which can be performed similarly to the probiotic tests (non-toxicity, antipathogenic activity, and functional properties) [
53], a negative cell viability test must be performed to guarantee the absence of viable cells. The most recommended test is direct plate cultivation without the use of several dilutions. Alternatives also include microscopies or real-time PCR, which may present obstacles due to the presence of cell debris and remaining genetic material in the solution [
58]. Additionally, further characterization of the postbiotic is recommended, such as evaluation of the saccharides, organic acids, antibiotics, bacteriocins, proteins, and enzymes. Monosaccharides, N-acetylglucosamine, alcohols, and organic acids can be detected through chromatographic methods, such as HPLC and thin layer [
59,
60]. For the antibiotics and bacteriocins, although they can be identified through chromatography and spectrometry, their activity is better tested with agar diffusion well tests against classical pathogens to verify if the existing biocides remain active [
61,
62]. As for the proteins, an interesting strategy is to characterize a proteomic to find unnamed proteins and enzymes as well, other than the regularly known ones [
63]. Enzymatic activity is difficult to detect in a general solution, yet it is possible to detect specific enzymes using nanokits or probes developed for the exclusive detection of their activity, such as the detection of sphingomyelinase through fluorescent nanoliposome [
64].
However, it is important to note that the industrial production of postbiotics should be carried out with caution. There is not enough data in the literature demonstrating the effect of scale-up on their biological functions, since all studies were performed on a laboratory scale. Therefore, it is still necessary to evaluate the influence of each step on the stability and biological activity of postbiotics.
3. Postbiotics Formulation
The composition of skin cosmetic products contains up to 20 different ingredients necessary to provide the intended efficacy, safety, and commercial acceptability. Water, surfactants, preservatives, barrier agents, enhancers, humectants, and active ingredients are the main key ingredients, and the categorization of the final product (e.g., lotion, cream, moisturizing) depends on the concentrations of these chemical compounds [
65]. Ultra-pure water is the basis of skin products, acting as the solvent basis to achieve the final consistency and contributing to the improvement of skin moisturizing levels [
66]. Surfactants are amphiphilic substances with the capability to reduce the surface between liquids with different polarities due to the presence of both hydrophobic and hydrophilic moieties in their chemical structure [
67], providing a homogeneous and uniform texture to the final product. Preservatives, such as parabens and formaldehyde, are substances able to prevent the growth of spoilage and harmful microorganisms, counteract the generation of reactive oxygen species (ROS), and impede the oxidation of cosmetics [
68]. Barrier agents reduce the water loss and direct contact to sensitizer, irritant, or allergen compounds that may be present on the formulae through the creation of a thin, hydrophobic layer over the skin. Enhancers, such as denatured alcohol, glycols, and esters, are added in order to improve the penetration of active ingredients through lipid fluidization, lipid extraction, and lipid ordering mechanisms [
69]. Humectants, as suggested, improve the hydration of the skin surface through the attraction of water from lower layers and posterior fixation in the stratum corneum via formation of hydrogen bounds [
65].
In addition to this ‘basic’ composition, synthetic-active ingredients—encompassing anti-inflammatory, corticosteroids, immunosuppressive, and antimicrobial agents—are also added to the formulation of skin products directed towards the treatment of skin disorders, such as acne vulgaris, psoriasis, atopic dermatitis, and wound healing [
70,
71,
72]. However, recent studies highlighted that prolonged exposition to these synthetic compounds as a sole treatment can result in numerous adverse events, including skin irritation, dryness, exfoliation, erythema, and striae [
73,
74]. As previously discussed, these reactions lead to a change in the structure of the microbial community, comprising the biological functions of the skin and severity increase in atopic dermatitis [
75,
76], acne vulgaris [
77,
78], and psoriasis [
79,
80]. This fact, allied to a market change to address the superior demand for green cosmetics, lead to the development of biocosmetics [
66].
Among the biotechnological solutions presented, the addition of postbiotics as active ingredients proved to be one of the most promising due to the absence of bacteremia and fungaemia risks, besides the inherent stability during industrial processes and shelf life [
9]. The formulation of cosmetic products containing postbiotics also have proven to be less costly, once it is unnecessary to maintain cell viability in the final product during transportation and storage. Golkar et al. [
81] proposed the formulation of cold creams for wound healing in animal models by the direct addition (0.01% [
w/
w]) of lyophilized supernatant obtained after centrifugation of
Lactobacillus fermentum,
L. reuteri, and
Bacillus subtilis ferments in the synthetic medium. Direct addition of LactoSporin
® (2% [
w/
w]), an extracellular, low-weight protein metabolite produced by the patented probiotic
B. coagulans MTCC 5856 [
82], have also been evaluated by Majeed et al. [
83] from the formulation of topical creams to the treatment of mild-to-moderate acne lesions.
Although cost-effective, the direct addition of postbiotics expose these substances to external conditions (e.g., UV light, temperature, pH, oxidation) in topical formulations, which may result in the loss of stability and biological activity. In this sense, the encapsulation with microparticles and nanoparticles is a viable option, providing the necessary protection and allowing the active ingredients to be retained in the top of epidermis [
84]. A recent study conducted by Ashoori et al. [
85] investigated the characterization and application of topical formulations, each containing 1 g of probiotic lysates from
L. reuteri,
L. fermentum, and
B. subtilis sp.
Natto in 100 g of chitosan nanogel (1%
w/
w), for wound healing in vivo. The characterization of the nanocapsules revealed spherical and uniform capsules with sizes ranging from 10 to 50 nm and appropriate physical stability, thus supporting the chitosan nanogel as an appropriate carrier for postbiotics in cosmetic formulae. Although it is a field recently investigated by science, cosmetic products containing postbiotics in their formulae are already available in the market with alleged claims and benefits, such as ProRenew Complex CLR™ NP (Onlystar Bio-Technology, Beijing, China), Bioptimized™ Guava (Innovacos, Mt. Arlington, NJ, USA), and PREBIOME™ (Radiant) [
7].
4. Applications and Effects of Postbiotics in the Treatment of Skin Condition
Table 1 shows the main advances related to in vivo evaluation of the efficacy of postbiotics associated with skin health. The biological properties of postbiotics have been explored by researchers to develop alternative treatments for alopecia, acne vulgaris, atopic dermatitis, and wound healing. The postbiotics are derived from well-defined microorganisms or a combination of microorganisms of the
Lactobacillus and
Bacillus genera (
Table 1). However, other bacterial (e.g.,
Bifidobacterium,
Lactococcus,
Leuconostoc,
Pediococcus, Weissella,
Enterococcus, and
Fructobacillus) and fungal (e.g.,
Citeromyce,
Cystofilobasidium,
Hanseniaspora,
Issatchenki,
Pichia, and
Saccharomyces) have already been described and characterized as probiotic microorganisms, and can still be explored as safe sources of postbiotics [
53]. In general, the application of postbiotics occurs by incorporating these molecules in the formulation of creams for topical use or by encapsulation and oral administration [
86,
87].
Table 1. In vivo evaluation of the efficacy of postbiotics for the treatment of diseases affecting the skin.
4.1. Alopecia
Alopecia areata (AA) is an autoimmune condition that affects the scalp, causing hair loss. In some more severe cases, AA can spread to the entire scalp (alopecia totalis) or even the body (alopecia universalis) [
90]. Due to these characteristics, AA is often associated with mental health problems, especially social anxiety [
91]. To date, there is no cure for AA [
92]. Treatment for AA includes topical or intralesional application of corticosteroids for mild cases to immunotherapy with diphenylcyclopropenone or squaric acid dibutylester for severe cases [
93]. However, during treatment, patients may experience some side effects, such as lymph node enlargement, blisters, headache, intense itching, weight gain, and hyperpigmentation [
92,
93,
94]. In addition, the long-term application can lead to a process of skin atrophy [
92].
Due to these conditions, some alternatives for the treatment of AA have been proposed and are under evaluation, such as low-level light therapy [
95], Janus Kinase (JAK) inhibitors [
96], and platelet-rich plasma (PRP) treatment, which is known as an ‘elixir’ for hair growth [
97]. PRP is usually obtained by centrifuging the patient’s own blood and used to formulate a gel for topical application. However, the mechanism of action of PRP treatment has not been fully elucidated, but it is speculated that several growth factors that are present in platelets, mainly polypeptides, act by stimulating hair follicle cell differentiation and proliferation as well as inhibiting the process of apoptosis [
98,
99,
100]. Although the use of PRP has shown great potential in several studies [
101,
102,
103], the treatment still has many limitations, as there is no standardization of the production process and platelet concentration, besides presenting low stability, which makes topical applications difficult [
88,
98].
To overcome these barriers, scientists have sought modern alternatives, which include the use of bioactive peptides obtained from biotechnological tools combined with postbiotics [
88,
98]. This approach aims to mimic the growth factors found in PRP treatment and thus opens avenues for new therapeutic approaches. For instance, a double-blind randomized study of 160 people was conducted to evaluate the efficacy of a gel formulation containing postbiotics for topical application for the treatment of AA. These volunteers were randomly divided into two groups: (i) the treatment group (received the gel TR-PRP plus-Cel) and (ii) the control group (control received placebo). Among the treated patients, about 47.50% showed a complete regression of hair loss, while 13.75% exhibited a partial regression and only 6.25% of treated patients reported no response. In the control group, only 5% of individuals reported complete regression [
88]. The positive effect of TR-PRP plus-Cels was associated with the postbiotic plantaricin A (PlnA) and
Lactobacillus kunkeei-fermented bee bread (bee bread). PlnA is a peptide that presents several biological properties, among them the ability to induce key mediators of epithelial cell proliferation, migration, and differentiation, while bee bread is known for its immunomodulatory role. In addition, both compounds exhibit high antioxidant and antimicrobial activity [
88,
104,
105,
106]. Rinaldi et al. [
88] speculated that the postbiotics present in TR-PRP plus-Cels may also positively modulate the structure of the scalp microbial community. However, further studies are still needed to evaluate the effect of microbial metabolites on the scalp and how these compounds can be used as an alternative method for and treatment of AA.
4.2. Acne Vulgaris
Acne vulgaris is considered a chronic inflammatory disease affecting the pilosebaceous unit (hair follicles in the skin associated with a sebaceous gland) present on the face, neck, chest, and coast [
107]. Acne is characterized by papules, nodules, comedones, pustules, cysts, and often scars on the affected area [
108,
109]. The pathogenesis of acne is not fully known, but some studies indicate that factors such as androgen-induced excessive sebum production, hyperkeratinization, obstruction of the sebaceous follicles, bacterial colonization of the hair follicles by
C. acnes, and inflammation are the main factors behind this disease [
107,
110]. Although acne is not a life-threatening disease, it can trigger a range of psychological problems that include anxiety, social isolation, and even suicidal intent [
109,
111,
112]. In general, there is no ideal treatment for acne, but topical therapies with retinoids, benzoyl peroxide, and antibiotics, when used in combination, show efficacy in mild and moderate cases. On the other hand, in severe cases, oral antibiotics combined with the topical use of benzoyl peroxide are generally recommended [
107,
113,
114].
Benzoyl peroxide has been used for the treatment of acne since 1934 and its mechanism consists of reducing the aerobic bacteria population by strong oxidation processes [
27]. Furthermore, benzoyl peroxide does not allow
C. acnes to develop resistance to antibiotics. Retinoids, on the other hand, can reduce the obstruction within the follicle, and are mainly used in cases where the patient presents comedonal and inflammatory acne. However, treatment with these compounds is usually associated with redness, irritation, dryness, and excessive flaking of the skin [
113,
115,
116].
Postbiotics have been shown to be a potential alternative in the treatment of acne [
117]. For instance, a study conducted by Majeed et al. [
83] compared the effect of LactoSporin
® (a filtered extract obtained from a fermented
Bacillus coagulans MTCC 5856) with benzoyl peroxide in 64 individuals diagnosed with mild and moderate acne. Both treatments showed significant improvements in dermatological assessment of closed and open comedones and papule count. However, the effect of postbiotics on closed comedones appeared earlier compared to benzoyl peroxide, showing an advantage over the standard treatment. Furthermore, the application of LactoSporin resulted in a significant decrease in sebum secretion, leading to a reduction in oiliness, spots, and redness around acne. This effect may be associated with the ability of the postbiotic to inhibit the enzyme 5-alpha reductase, as shown in in vitro tests [
118], as 5-alpha reductase plays an important role during the production of hormones that stimulate sebaceous gland secretion favoring the growth of
C. acnes [
119]. In addition, LactoSporin has been shown to have microbial activities against
P. aeruginosa,
S. aureus,
S. epidermidis, and, most importantly, it was found to be effective against
C. acnes. In addition, it was able to inhibit the formation of microbial biofilms [
83,
118].
Further studies involving the characterization and application of novel postbiotics for the treatment of acne vulgaris are still under development. An interesting approach was recently suggested by Chung et al. [
120] and consists of the development of a postbiotic complex (PC), i.e., the use of two or more strains. This strategy has great potential, because it is difficult for a single strain to present all the desired biological properties. As the biological activities of various probiotics are known, it is possible to obtain postbiotics from these different strains and formulate new products with the desired characteristics, thus increasing their range of application. For example, Chung et al. [
120] evaluated in vitro the biological activity of a CP produced from
Lactobacillus helveticus HY7801
Lactobacillus lactis HY449. The results showed that the CP derived from these strains showed an antibacterial effect against
S. aureus and
C. acnes, in addition to anti-inflammatory activity, regulating the levels of inflammatory cytokines and hyaluronic acid in keratinocytes. The authors also evaluated the main metabolites present in CP and attributed these biological properties to the molecule’s hypoxanthine, 2-hydroxyisocaproic acid, succinic acid, ornithine, and GABA. However, it is still necessary to formulate products containing these potential postbiotics and to prove their efficacy through in vivo tests.
4.3. Atopic Dermatitis
Atopic dermatitis (AD) is a chronic inflammatory skin disease characterized by the presence of pruritic eczema. Although this disease is more common in children, adults are also affected [
121,
122,
123]. Several studies have shown that the pathophysiology of AD is mainly associated with a process of immune system dysregulation, loss of skin barrier efficiency, microbial dysbiosis of the skin, and the itch/scratch cycle [
122,
124,
125,
126]. However, the literature suggests that problems in the epidermal barrier are the main factor responsible for AD. It is speculated that these alterations may be associated mainly with genetic/epigenetic and environmental factors [
127,
128]. Treatment of mild to moderate AD usually includes the use of corticosteroids, topical emollients, and calcineurin inhibitors. About 20% of patients present moderate to severe symptoms, making topical treatments not very effective due to the marked inflammation process [
129,
130]. Generally, these patients undergo phototherapy and treatment with systemic immune modulators [
130]. However, these treatments are associated with significant adverse effects and do not offer long-term “cures”, while other patients may show resistance to these standard treatments [
90,
128].
Therefore, the use of probiotics and postbiotics has been proposed as an alternative treatment for AD [
87,
89,
131]. For example, a double-blind placebo-controlled study showed that administration of heat-treated milk fermented by
L. acidophilus L-92 exhibited immunoregulatory properties, as it was able to attenuate the symptoms of Japanese cedar pollen allergy [
132]. Later, Hong et al. [
89] and Inoue et al. [
87] showed that the administration of both viable and heat-inactivated
L. acidophilus L-92 also resulted in a significant improvement in AD symptoms in adults and children. In both studies, the improvement of patients treated with the L-92 strain was mainly associated with the suppression of Th2 dominant inflammation. In addition, no serious side effects were observed, showing that, at this early-stage, L-92 can be used as a food supplement to reduce the dose of steroidal anti-inflammatory ointments needed for atopic treatment.
In general, several other studies also show biological activity, demonstrating the potential of
L. acidophilus L-92 to be applied as postbiotics [
133,
134,
135,
136]. However, a detailed study of the metabolic profile of this strain is needed to identify the molecules that may be associated with the beneficial effects observed by the authors [
87,
89,
131]. These findings may open new avenues for the development of topical ointments for the treatment of mild and moderate cases, because, in addition to efficacy, postbiotics tend to present fewer side effects to the skin, thus being able to replace or reduce traditional treatments.
4.4. Wound Healing
The skin is the largest organ in the body and to perform its regulatory and barrier functions it needs to be intact. Skin breakdowns due to injury, disease, or operation are defined as wounds [
137]. Although this organ can restore its integrity spontaneously, wound care is extremely important, mainly for the protection of the open site, preventing infection, dryness, and relieving pain [
138]. Classically, this process of wound healing is divided into four distinct phases: hemostasis, inflammation, proliferation, and tissue remodeling. Several factors can slow or impair the healing process, among them microbial proliferation in the wound [
139]. Microbial growth and maintenance of these microorganisms in wounds are usually associated with biofilm production. In advanced stages, there is a risk that the immune system will be unable to contain this infection. This process can result in a severe clinical picture of marked inflation or purulence [
140].
Therefore, research is needed to develop new therapeutic agents that can assist in wound treatment, decreasing the infection rate, and accelerating the healing process. Postbiotics are valuable compounds as they have several biological properties (e.g., immunomodulatory, anti-inflammatory, antimicrobial, and angiogenic) that can favor wound healing. Recently, Golkar et al. [
81] conducted a study in rats to evaluate the healing action of three new ice creams containing postbiotics derived from
L. fermentum ATCC 9338,
L. reuteri ATCC 23272 and
B. subtilis sp.
natto ATCC 15245. Although the three formulations showed a significant improvement in the healing process compared to the control, ice cream containing postbiotics derived from
B. subtilis sp.
natto showed more promising results. In general, this group presented a higher content of hydroxyproline, a basic component found in collagen. Thus, the increase in hydroxyproline is used as a parameter to assess the amount of collagen that has been produced, indicating greater progression in the healing process. Furthermore, histopathological analyses also revealed that the cream containing the postbiotic
B. subtilis sp.
natto showed less degree of inflammation while a mild fibrosis process was observed in all groups except the untreated group, which resulted in moderate fibrosis [
81]. These studies provide insights for the development of new postbiotic-based products to be considered as an alternative treatment in wound healing.
This entry is adapted from the peer-reviewed paper 10.3390/fermentation9030264