This review discusses two topics: (i) the prognostic impact of tumor-infiltrating lymphocytes and (ii) predictive biomarkers for immune checkpoint inhibitors, to shed light on lymphocyte migration in four solid tumors, the urothelial carcinoma, renal cell carcinoma, prostate cancer, and retroperitoneal sarcoma.
- tumor-infiltrating lymphocyte
- immune cells
- tumor microenvironment
- prognosis
- immune checkpoint inhibitor
- treatment response
- urothelial carcinoma
- renal cell carcinoma
- prostate cancer
- retroperitoneal sarcoma
Dear author, the following contents are excerpts from your papers. They are editable.
(If you are interested in this work, please check and revise and then submit. A good entry will better present your ideas and findings to other scholars. Readers will also be able to access your paper directly through entries. No worry about the format, we'll correct it after your submission)
1. Introduction
The interaction and cross-talk among tumor cells and several immune cells in a tumor microenvironment are dynamic and complex processes [1,2]. In 2011, Hanahan and Weinberg defined “avoiding immune destruction” and “tumor-promoting inflammation” as emerging cancer hallmarks [3], which are host-dependent biological characteristics play crucial roles in the immune cell-mediated orchestration of tumor proliferation, progression, angiogenesis, epithelial-to-mesenchymal transition (EMT), invasion, and metastasis [4,5]. Although inflammation caused by the innate immune system was originally designed to fight infections and heal wounds, “tumor-promoting inflammation” can inadvertently contribute to multiple cancer hallmark capabilities by supplying active molecules to the tumor microenvironment [3,6,7,8]. “Avoiding immune destruction” allows tumor cells to escape immunosurveillance; the main role of T lymphocytes, B lymphocytes, macrophages, natural killer (NK) cells, neutrophils, and dendritic cells [3,9,10].
An increasing body of oncological research has provided evidence to suggest that tumor-infiltrating lymphocytes (TILs), including T lymphocytes such as CD8+ T cells and regulatory T cells, and NK cells such as tumor-associated NK cells and tumor-infiltrating NK cells, are double-edged swords in cancer [9,11,12]. The density, composition, and types of TILs vary greatly across tumor stage [10] and tumor entity [13]. Moreover, these features display significant heterogeneity between patients with the same type of tumors [14,15]. Lymphocyte migration to neoplastic lesions is mainly controlled by chemotactic factors, including chemokines and small cytokines, which are secreted from immune cells and tumor cells [16]. There are three types of immunological profiles: (1) immunologically “tumors” present with a high degree of T cell infiltration (e.g., melanoma, non-small-cell lung carcinoma, and renal cell carcinoma (RCC)) [17,18]; (2) immunologically “cold tumors” present with scarce immune infiltrates (e.g., prostate cancer (PCa) and pancreatic cancer) [19,20]; (3) immunologically “altered tumors” in which peri-tumoral sites are densely inflamed with immune cells which lack the capability to infiltrate the tumor [21]. Classification of tumors according to their immune phenotype can help predict responses to immune checkpoint inhibitors (ICIs), such as anti-programmed cell death 1 (PD-1) inhibitors, pembrolizumab, and nivolumab. Higher levels of immune cell infiltration and interferon signature (a T-cell-inflamed phenotype) are associated with a positive response to ICIs [19]. However, many other solid tumors fail to respond to ICIs due to limited immunogenicity, unfavorable tumor microenvironments with scarce immune infiltrates, and excessive accumulation of regulatory T cells [17]. Therefore, the potential to develop new therapeutic approaches that can convert immunologically ’cold’ or ’altered’ environments to ’hot’ environments has recently attracted increasing attention. [22,23].
As immune contexture can vary widely across types of tumor and tumor microenvironment, there exists a significant lack of clinically available definitive biomarkers that provide accurate predictions for treatment responses, especially in urogenital malignancies. Additionally, in this review, we will discuss the functional heterogeneity in the tumor-infiltrating immune system to explore its prognostic impact after surgery and other treatments. This review focuses on two main topics: (i) the prognostic impact of TILs and (ii) predictive biomarker for ICIs, to shed light on lymphocyte migration in four solid tumors which are urothelial carcinoma (UC), RCC, PCa, and retroperitoneal sarcoma (RSar) (Figure 1).
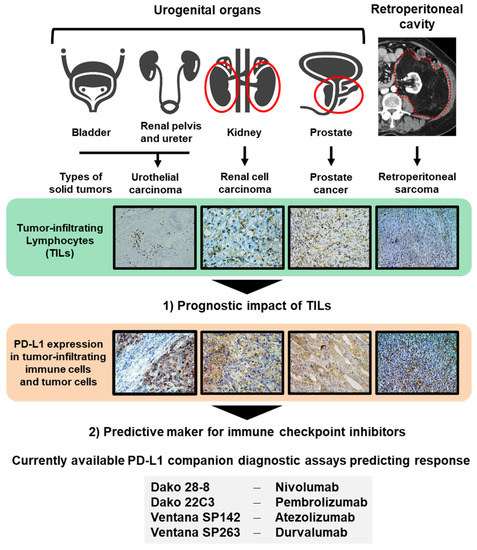
Figure 1. Two topics discussed in this review. Urologists should handle several malignancies arising from different organs, including the bladder, renal pelvis, ureter, kidney, prostate, and tissues of the retroperitoneal cavity. In this review, we discuss two topics: (1) the prognostic impact of tumor-infiltrating leukocytes (TILs) and (2) predictive markers for immune checkpoint inhibitors to shed light on lymphocyte migration in four solid tumors, the urothelial carcinoma, renal cell carcinoma, PCa, and retroperitoneal sarcoma. Currently available PD-L1 companion diagnostic assays predicting response to immune checkpoint inhibitors are shown.
2. Limitations and Current Perspective Regarding the Assessment of TILs
Researchers focused exclusively on the potential of TILs as a prognostic or predictive marker. Relevant studies have been performed in a retrospective manner and in relatively small cohorts. The definitions of TILs, such as inclusion of intratumoral TILs and/or stromal TILs, and the scoring methodology varied among studies. These inconsistencies hinder comparisons across studies and extrapolation of findings to clinical practice. Large studies investigating the potential prognostic value of TILs as assessed on HE staining are lacking. International Immuno-Oncology Biomarker Working Group on Breast Cancer has developed the international guidelines regarding the assessement of TILs on HE-stained slides without any specific staining [109]. The purpose of this group is to develop standards on the assessment of immuno-oncology biomarkers to aid pathologists, clinicians and researchers in their research and daily clinical practice. International Guidelines on TIL-assessment in solid tumors Part 2 provided the recommendation in assessment of genitourinary carcinomas. According to the guidelines [109], separate reporting of intratumoral TILs and stromal TILs is recommended—this is based on the context of atezolizumab treatment in mUC, where the PD-L1 “immune cell” score is derived from the stromal TILs score [110]. In addition, special care should be taken to avoid areas of tumor zones with necrosis, coagulation artifact, and previous biopsy sites, which is a common finding in resected specimens of bladder tumor. However, detailed tutorial for RCC, PCa, and sarcoma is not available because of insufficient data to make specific recommendations.
Early data showed the presence of TILs in UC was associated with a favorable prognosis [111]. As to early data of RCC, increased TILs, both CD4-positive and CD8-positive T cells, appear to related with high risk of post-nephrectomy recurrence and poor prognosis [112,113,114,115,116]. However, accumulating evidence, largely based on IHC quantification of different TIL subsets, have somehow turned conflicting results on the prognostic relevance of TILs (Table 1). Majority of reports on TILs in PCa have focused on the prognostic value of TILs, while few studies investigating the potential to predicting response to drug therapies. Most reports have shown the evidence for a relationship between the high TILs and increased risk of recurrence [117,118,119], metastasis [120], and poor cancer specific survival [121]. The result on the composition of TILs in are heterogeneous and sometimes conflicting, and the relationship between TILs and survival is still unclear in PCa [109]. Overall, we emphasis on the importance of uniform assessment of TILs and uniform comparison of study results in research practices.
3. Conclusions
As, an “immunotherapy tsunami”, in particular ICIs, has overtaken the oncological field in this decade, it is mandatory for Physicians to deepen knowledge about cancer immunity and tumor immune microenvironment. This review highlights comprehensively the following two topics: (i) prognostic impact of TILs and (ii) predictive maker for ICIs in four urological solid tumors: UC, RCC, PCa, and Sar. Although there is accumulating evidence that the density of TILs can serve as a prognostic biomarker and/or predictive biomarker for immunotherapies, inconsistency of TIL evaluation and interpretation for the results seems to hinder its clinical application. Unfortunately, across different solid malignancies, the response rate and predictive markers for ICIs may vary significantly. Multiple biomarkers including tumor-infiltrating immune cells, PD-L1 expression, other immune checkpoint protein expression, mRNA gene expression analysis, mismatch-repair deficiency, and tumor mutational burden may need to overcome disease heterogeneity and complex tumor immunity. Both identification of positive or negative predictive biomarkers of ICIs and development promising combination are required urgently to refine the clinical management of advanced urological malignancies. Further studies with large-scale cohorts and long follow-up periods to prove the clinical impact of novel prognostic/predictive biomarkers, followed by their adoption in clinical practice.
reference
- Chen, D.S.; Mellman, I. Oncology meets immunology: The cancer-immunity cycle. Immunity 2013, 39, 1–10.
- Chen, D.S.; Mellman, I. Elements of cancer immunity and the cancer–immune set point. Nature 2017, 541, 321–330.
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674.
- Bruno, A.; Pagani, A.; Magnani, E.; Rossi, T.; Noonan, D.M.; Cantelmo, A.R.; Albini, A. Inflammatory angiogenesis and the tumor microenvironment as targets for cancer therapy and prevention. Cancer Treat. Res. 2014, 159, 401–426.
- Piotrowski, I.; Kulcenty, K.; Suchorska, W. Interplay between inflammation and cancer. Rep. Pract. Oncol. Radiother. 2020, 25, 422–427.
- Frost, F.G.; Cherukuri, P.F.; Milanovich, S.; Boerkoel, C.F. Pan-cancer RNA-seq data stratifies tumours by some hallmarks of cancer. J. Cell. Mol. Med. 2020, 24, 418–430.
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899.
- Qian, B.Z.; Pollard, J.W. Macrophage diversity enhances tumor progression and metastasis. Cell 2010, 141, 39–51.
- Shembrey, C.; Huntington, N.D.; Hollande, F. Impact of Tumor and Immunological Heterogeneity on the Anti-Cancer Immune Response. Cancers 2019, 11, 1217.
- Bindea, G.; Mlecnik, B.; Tosolini, M.; Kirilovsky, A.; Waldner, M.; Obenauf, A.C.; Angell, H.; Fredriksen, T.; Lafontaine, L.; Berger, A.; et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 2013, 39, 782–795.
- Lakshmi Narendra, B.; Eshvendar Reddy, K.; Shantikumar, S.; Ramakrishna, S. Immune system: A double-edged sword in cancer. Inflamm. Res. 2013, 62, 823–834.
- Bassani, B.; Baci, D.; Gallazzi, M.; Poggi, A.; Bruno, A.; Mortara, L. Natural Killer Cells as Key Players of Tumor Progression and Angiogenesis: Old and Novel Tools to Divert Their Pro-Tumor Activities into Potent Anti-Tumor Effects. Cancers 2019, 11, 461.
- Gentles, A.J.; Newman, A.M.; Liu, C.L.; Bratman, S.V.; Feng, W.; Kim, D.; Nair, V.S.; Xu, Y.; Khuong, A.; Hoang, C.D.; et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med. 2015, 21, 938–945.
- Doucette, T.; Rao, G.; Rao, A.; Shen, L.; Aldape, K.; Wei, J.; Dziurzynski, K.; Gilbert, M.; Heimberger, A.B. Immune Heterogeneity of Glioblastoma Subtypes: Extrapolation from the Cancer Genome Atlas. Cancer Immunol. Res. 2013, 1, 112–122.
- Reuben, A.; Gittelman, R.; Gao, J.; Zhang, J.; Yusko, E.C.; Wu, C.J.; Emerson, R.; Zhang, J.; Tipton, C.; Li, J.; et al. TCR Repertoire Intratumor Heterogeneity in Localized Lung Adenocarcinomas: An Association with Predicted Neoantigen Heterogeneity and Postsurgical Recurrence. Cancer Discov. 2017, 7, 12–17.
- Miyake, M.; Hori, S.; Morizawa, Y.; Tatsumi, Y.; Nakai, Y.; Anai, S.; Torimoto, K.; Aoki, K.; Tanaka, N.; Shimada, K.; et al. CXCL1-Mediated Interaction of Cancer Cells with Tumor-Associated Macrophages and Cancer-Associated Fibroblasts Promotes Tumor Progression in Human Bladder Cancer. Neoplasia 2016, 18, 636–646.
- Rodallec, A.; Sicard, G.; Fanciullino, R.; Benzekry, S.; Lacarelle, B.; Milano, G.; Ciccolini, J. Turning cold tumors into hot tumors: Harnessing the potential of tumor immunity using nanoparticles. Expert Opin. Drug Metab. Toxicol. 2018, 14, 1139–1147.
- Kawashima, A.; Kanazawa, T.; Goto, K.; Matsumoto, M.; Morimoto-Okazawa, A.; Iwahori, K.; Ujike, T.; Nagahara, A.; Fujita, K.; Uemura, M.; et al. Immunological classification of renal cell carcinoma patients based on phenotypic analysis of immune check-point molecules. Cancer Immunol. Immunother. 2018, 67, 113–125.
- Vareki, S.M. High and low mutational burden tumors versus immunologically hot and cold tumors and response to immune checkpoint inhibitors. J. Immunother. Cancer 2018, 6, 157.
- Strasner, A.; Karin, M. Immune infiltration and prostate Cancer. Front. Oncol. 2015, 5, 128.
- Camus, M.; Tosolini, M.; Mlecnik, B.; Pagè, F.; Kirilovsky, A.; Berger, A.; Costes, A.; Bindea, G.; Charoentong, P.; Bruneval, P.; et al. Coordination of Intratumoral Immune Reaction and Human Colorectal Cancer Recurrence. Cancer Res. 2009, 69, 2685–2693.
- Prendergast, G.C.; Mondal, A.; Dey, S.; Laury-Kleintop, L.D.; Muller, A.J. Inflammatory Reprogramming with IDO1 Inhibitors: Turning Immunologically Unresponsive ’Cold’ Tumors ’Hot’. Trends Cancer 2018, 4, 38–58.
- Galluzzi, L.; Buqué, A.; Kepp, O.; Zitvogel, L.; Kroemer, G. Immunological Effects of Conventional Chemotherapy and Targeted Anticancer Agents. Cancer Cell 2015, 28, 690–714.
- Hendry, S.; Salgado, R.; Gevaert, T.; Russell, P.A.; John, T.; Thapa, B.; Christie, M.; Van De Vijver, K.; Estrada, M.V.; Gonzalez-Ericsson, P.I.; et al. Assessing Tumor-Infiltrating Lymphocytes in Solid Tumors: A Practical Review for Pathologists and Proposal for a Standardized Method from the International Immuno-Oncology Biomarkers Working Group: Part 2: TILs in Melanoma, Gastrointestinal Tract Carcinomas, Non-Small Cell Lung Carcinoma and Mesothelioma, Endometrial and Ovarian Carcinomas, Squamous Cell Carcinoma of the Head and Neck, Genitourinary Carcinomas, and Primary Brain Tumors. Adv. Anat. Pathol. 2017, 24, 311–335.
- Galsky, M.D.; Arija, J.Á.A.; Bamias, A.; Davis, I.D.; De Santis, M.; Kikuchi, E.; Garcia-Del-Muro, X.; De Giorgi, U.; Mencinger, M.; Izumi, K.; et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): A multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2020, 395, 1547–1557.
- Sharma, P.; Shen, Y.; Wen, S.; Yamada, S.; Jungbluth, A.A.; Gnjatic, S.; Bajorin, D.F.; Reuter, V.E.; Herr, H.; Old, L.J.; et al. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc. Natl. Acad. Sci. USA 2007, 104, 3967–3972.
- Kolbeck, P.C.; Kaveggia, F.F.; Johansson, S.L.; Gune, M.; Taylor, R. The relationships among tumor-infiltrating lymphocytes, histopathologic findings, and long-term clinical follow-up in renal cell carcinoma. Mod. Pathol. 1992, 5, 420–425.
- Bromwich, E.J.; McArdle, P.A.; Canna, K.; McMillan, D.; McNicol, A.; Brown, M.; Aitchisonet, M. The relationship between T-lymphocyte infiltration, stage, tumour grade and survival in patients undergoing curative surgery for renal cell cancer. Br. J. Cancer. 2003, 89, 1906–1908.
- Remark, R.; Alifano, M.; Cremer, I.; Lupo, A.; Dieu-Nosjean, M.C.; Riquet, M.; Crozet, L.; Ouakrim, H.; Goc, J.; Cazes, A.; et al. Characteristics and Clinical Impacts of the Immune Environments in Colorectal and Renal Cell Carcinoma Lung Metastases: Influence of Tumor Origin. Clin. Cancer Res. 2013, 19, 4079–4091.
- Nakano, O.; Sato, M.; Naito, Y.; Suzuki, K.; Orikasa, S.; Aizawa, M.; Suzuki, Y.; Shintaku, I.; Nagura, H.; Ohtani, H. Proliferative Activity of Intratumoral CD8+ T-Lymphocytes As a Prognostic Factor in Human Renal Cell Carcinoma. Cancer Res. 2001, 61, 5132–5136.
- Hotta, K.; Sho, M.; Fujimoto, K.; Shimada, K.; Yamato, I.; Anai, S.; Konishi, N.; Hirao, Y.; Nonomura, K.; Nakajima, Y. Prognostic significance of CD45RO+ memory T cells in renal cell carcinoma. Br. J. Cancer. 2011, 105, 1191–1196.
- Irani, J.; Goujon, J.M.; Ragni, E.; Peyrat, L.; Hubert, J.; Saint, F.; Mottet, N. High-grade inflammation in prostate cancer as a prognostic factor for biochemical recurrence after radical prostatectomy. Urology 1999, 54, 467–472.
- Karja, V.; Aaltomaa, S.; Lipponen, P.K.; Isotalo, T.; Talja, M.; Mokka, R. Tumour-infiltrating Lymphocytes: A Prognostic Factor of PSA-free Survival in Patients with Local Prostate Carcinoma Treated by Radical Prostatectomy. Anticancer Res. 2005, 25, 4435–4438.
- Zeigler-Johnson, C.; Morales, K.H.; Lal, P.; Feldman, M. The Relationship between Obesity, Prostate Tumor Infiltrating Lymphocytes and Macrophages, and Biochemical Failure. PLoS ONE 2016, 11, e0159109.
- Richardsen, E.; Uglehaus, R.D.; Due, J.; Busch, C.; Busund, L.T. The prognostic impact of M-CSF, CSF-1 receptor, CD68 and CD3 in prostatic carcinoma. Histopathology 2008, 53, 30–38.
- McArdle, P.A.; Canna, K.; McMillan, D.C.; McNicol, A.M.; Campbell, R.; Underwood, M.A. The relationship between T-lymphocyte subset infiltration and survival in patients with prostate cancer. Br. J. Cancer 2004, 91, 541–543.
This entry is adapted from the peer-reviewed paper 10.3390/cancers12113153
