Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The nutritional composition of silkworms will likely have multiple implications for humans, animals, and the environment. The silkworm pupae attracted interest due to lipids and protein profiles. Furthermore, the valuable level of the essential fatty acids (alpha-linolenic and linoleic from the n-3 and n-6 family) results in significant physiological functions in the human body that support good health.
- silkworm pupae
- B. mori
- oil
1. Introduction
The quality and quantity of fats consumed significantly impacts the aetiology of certain diseases. Numerous specialists have focused on recommended guidelines for the quantity and type of fat to consume. The primary constituent of lipid structure is straight-chain aliphatic carboxylic acids, known as fatty acids (FA). The most natural FA range from C4 to C22 [1]. Medical and scientific studies demonstrated the effectiveness of polyunsaturated FA (PUFA) in preventing and treating several diseases, including improved insulin sensitivity, reduced blood pressure, decreased thrombotic tendency, anti-inflammatory and antiarrhythmic effects, improved vascular endothelial function, and increased plaque stability [2][3][4]. PUFA act through receptors that regulate various cellular and metabolic processes, including adipogenesis, inflammation, oxidative stress, and the metabolism of lipids and glucose connected to energy homeostasis [5].
PUFA are mainly composed of n-6 (linoleic acid, C18:2n-6) and n-3 (alpha-linolenic acid, C18:3n-3), from which derive other long-chain PUFAs [6]. Our body lacks the necessary enzymes to synthesize PUFAs with important implications for life and thus they must be received from the diet. Essential fatty acids (EFAs), respectively alpha-linolenic and linoleic FA, have been regarded as nutraceuticals and functional foods [6]. On the other hand, n-3 FA has gained attention as a possible preventive and therapeutic agent to lower the risk of certain diseases due to its influence on the pathways involved in atherosclerosis and myocardial infarction.
Plant-based edible oils are becoming increasingly well-liked today due to their usefulness and health-enhancing qualities [4][7]. Vegetable oils, which include all saturated FA (SFA), monounsaturated FA (MUFA), and PUFA families, are sources of essential FA (EFA). However, the source plant or the technological procedure used to extract oil determines the content of their FA. Most of the investigated vegetable oils have higher PUFA content, notably higher n-6 PUFA concentration; however, some have a higher composition in beneficial n-3 PUFA, such as linseed, hempseed, camelina, pumpkin and sesame oil etc. [6][8][9][10][11][12]. The primary issues are related to the low worldwide production and ineffective technologies for processing edible oil from plants [13].
A sustainable alternative source of n-3-rich PUFA, which is recommended to be considered, is silkworm pupae (SP). Besides the excellent protein content of SP (about 55.6% as dry matter basis, DM), the fat concentration (about 32% as DM basis) can have higher importance. SP is considered a safe and valuable source of beneficial n-3 PUFA. As a result, SP oil is viewed as a reliable source with a variety of applications, such as in the food and pharmaceutical sectors, having anti-inflammatory action or stimulant effect on lymphatic circulation, and can be used in medicine as a treatment of sinusitis, otitis, bronchitis, asthma, tuberculosis, urinary infections, and post-surgery, and can also have possible cosmetics applications [14]. Both n-3 and n-6 FA, present in SP oils as primary components, are crucial for treating diabetes and cardiovascular disorders.
Bombyx mori (B. mori) is an excellent lepidopteran species representative for numerous scientific investigations, one of the most important insects from an economic point of view, but also as a model organism for medicine that is utilized in many developing nations. More than 3000 strains have been created and kept up over the lengthy period of domestication. According to Mokaya et al. [15] B. mori produce about 90% of the world’s silk. The silkworm is characterized by a short and rapid life cycle with four stages (egg, larvae, cocoon/pupa, and adult moth), as seen in Figure 1.
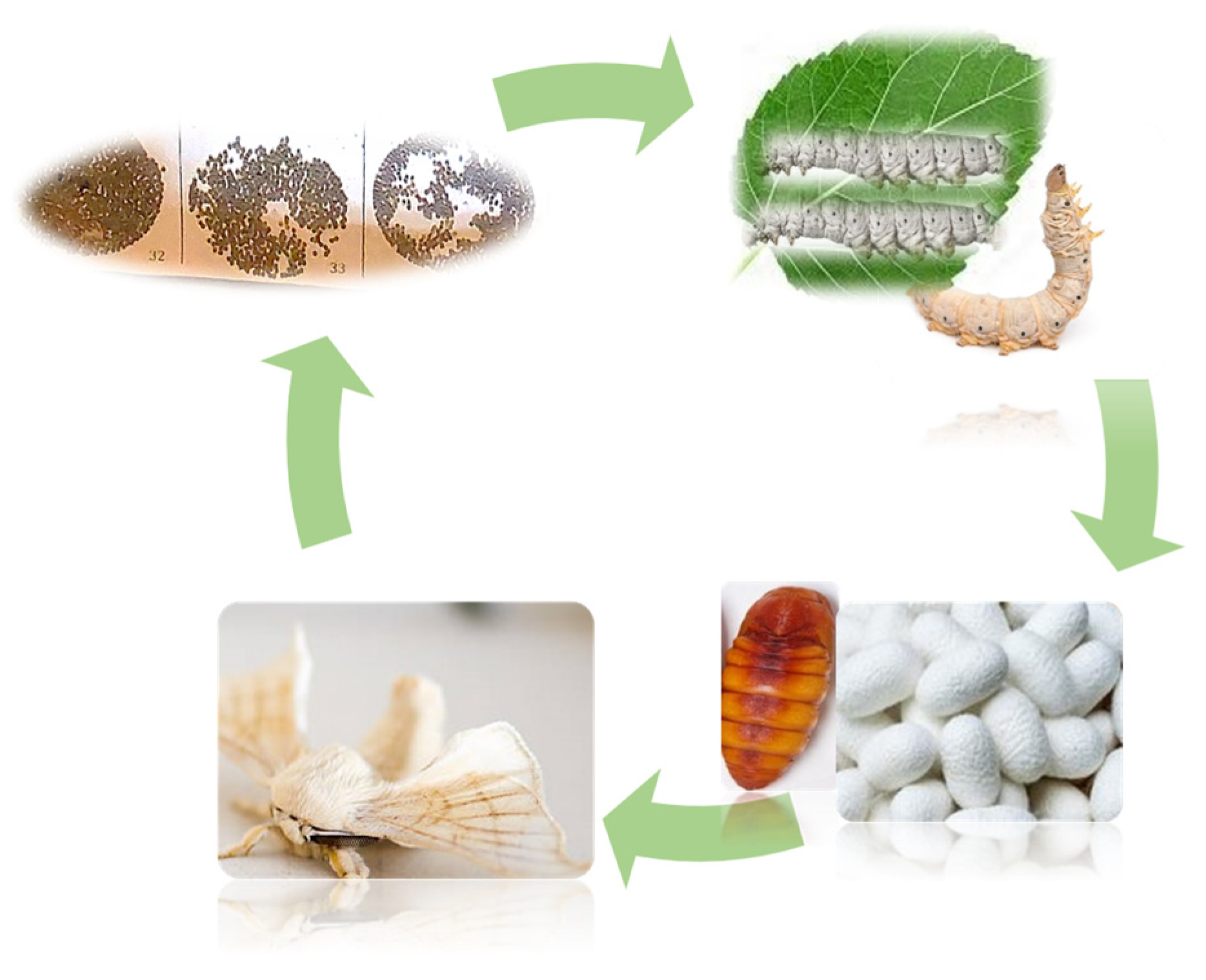
Figure 1. The life cycle of silkworm B. mori.
In Romania and other European countries, the most frequently used and raised mulberry silkworm is also the subject of extensive research. However, Antheraea pernyi, currently used primarily as a source of insect food and for cosmetic purposes, and Philosamia ricini (P. ricini), recognized for its white or brick-red eri silk, which is widely used in Brazil, China, and India, are important as well. In light of these considerations, this review focused on B. mori, aiming to summarize the FA composition of SP and their importance that associated with other bioactive compounds could influence SP value and diversify the ways of valorization and impact the environment.
Sericulture explores possibilities for the manufacturing of important biomaterials as well as utilization in regenerative medicines, tissue engineering, medical materials, drug delivery systems, cosmeceuticals, and food additives. Large amounts of waste are produced from the primary and secondary activities in the agro-industrial supply chain. The principal waste from the sericulture industry is SP, obtained after reeling the silkworm cocoons. These by-products can be discarded by the textile and feed, and food industries. Large-scale pupae disposal can have detrimental environmental effects in regions where silk is produced.
SP, the most valuable by-product, gave rise to the possibility of applications in medicine, nutrition, cosmetics, animal feed, fertilizer, etc.
Ordoñez-Araque et al. [16] proposed a change in mentality by adopting insect-based feeds instead of plant-based feeds as a first step to minimizing the environmental impact that traditional animal-based diets indirectly produce. This led as well to a considerable decrease in feed costs.
2. Application for Humans
Since the importance of insects as the final untapped biological resource began to be reconsidered in recent years, the insect industry’s expansion has been accelerating. SP, due to its high nutritional value, have the potential to positively affect health by fortifying human and animal diets with valuable bioactive compounds.
Although the protein has a higher concentration in DM, the oil structure also recommends this by-product for use in the medical and food industries. Numerous metabolic-related diseases have been connected to increased cellular oxidation, blood glucose, and blood pressure. The intracellular reactive oxygen species (ROS) regulate several signaling pathways against inflammation to activate an immune response. An imbalance of ROS can damage cells, leading to cardiovascular disease. The production of ROS is positively correlated with PUFA levels [17]. FAs regulate many different physiological processes [18]. The vital role of PUFA in treating a wide range of illnesses and disorders, such as diseases of the central nervous system, hypertension, inflammatory and immune disorders, depression and neurological dysfunction, and visual function, has been extensively discussed in the literature [18][19][20][21][22]. The EFAs content of SP is bioactive compounds associated with polyphenols, amino acids, and other nutritional compounds that have effects of antioxidant, anti-apoptotic, anti-genotoxic, anticancer, cardiovascular protective, and hepato-protective properties [19]. The pupae have gradually undergone additional processing to extract minerals and active substances, and they are now utilized in food modification and pharmaceutical investigations [23].
The pharmacological functions of SP oil were well summarized by Zhou et al. [19], who evaluated several research studies. Numerous biological actions of SP oil have been linked to improved blood circulation. By enriching SP oil with sodium salt solution through an esterification and saponification process, Kim et al. [24] demonstrated a beneficial effect as a meal and medication against vascular problems. Two different insects, Hermetia illucens (H. illucens, black soldier fly), fed with leftover fruits and vegetables and B. mori, fed mulberry leaves, were studied by Saviane et al. [25] for their oil antibacterial characteristics. They found an antibacterial effect with the same effectiveness, probably due to UFAs, typical of the SP. Related to what Saviane et al. [25] mentioned, the FAs have been found to impact Gram-positive bacteria, whilst very few species of Gram-negative bacteria are sensitive to FAs. This supports the theory that FAs are likely involved in the action mechanism of the oils. Long-chain UFAs, such as oleic, linoleic, and linolenic acids, have more antibacterial activity than SFA, such as palmitic and stearic acid, which are less effective. The antibacterial properties involve UFA in the defense system protecting against numerous pathogens to treat bacterial infections, with a positive impact on health.
Long-chain UFAs are prevalent in B. mori oil, mostly taken from the vegetal source used as a substrate for the larvae’s rearing. These FAs are also in higher amounts in H. illucens oil, particularly oleic acid. Furthermore, UFA are believed to have the ability to limit fat storage by speeding lipid metabolism. Its growing impact on fat metabolism-related proteins suggests that it can be used as a nutritional supplement.
The resulting increased lipid metabolism might also be suggested as a dietary supplement to prevent metabolic disorders [26]. Supplementing the human diet with SP oil may be an effective way to treat acute liver injury induced by an overdose of acetaminophen [27]. By adding SP oil to the diet, the area of gastric ulcer and secretion decreases; however, it raises gastric pH [28]. The oil content of SP ameliorates oxidative damage and reduces inflammations. It seems possible that eating SP could prevent Alzheimer’s disease due to the action of substances with antioxidant characteristics that decrease malondialdehyde concentration in the hippocampus. Inhibition of acetylcholinesterase can occur as well [29]. Certain peptides or amino acids play antioxidant functions. SP may have neuroprotective benefits by activating antioxidant enzymes and the cholinergic system. Therefore, SP may have therapeutic and preventative functions for neurodegenerative disorders [30]. It has been demonstrated in vitro the tyrosinase inhibitory, and free radical scavenging activities of oils and sericin of SP extracted from native silkworms (B. mori) [31].
Nutritionists claim that because SP are rich in calcium and phosphorus, two nutrients crucial for children’s growth, this food will be able to shield them from rickets and malnutrition.
Chitin present in SP skin (approximately 4% of DM) can be transformed into several beneficial substances, including chitosan, chitin sulfate, chitin nitrate, chitin xanthate, and sodium carboxymethyl chitin [32]. Chitin and chitosan derivatives are used in wound dressing, controlled release of drugs, and contact lenses. After low-temperature crystallization, chrysalis oil from SP is similar to linseed oil [32][33].
Protein hydrolysates from SP decreased nitric oxide production. A few proteins in SP hemocytes and hemolymph were found to have anti-inflammatory effects by lowering nitric oxide production [34].
An antigenotoxic activity of SP was mentioned by Deori et al. [35]. The presence of polyphenolic groups and FA-like linoleic acid may explain this.
Excreta have been used therapeutically in traditional Asian medicine to cure infectious disorders, headaches, and abdominal pain and reduce LDL cholesterol and blood pressure [36]. Silkworm excreta could have strong antioxidant activity due to its abundance of flavonoids, chlorophyll, alkaloids, carotenoids, and lutein components.
As a precaution, it should be highlighted that the SP of B. mori can produce certain anti-nutrients such as phytate, phytic phosphorus, as well as tannic acid, alkaloid, flavonoids, saponin, and oxalate. These anti-nutrients are present but at low concentrations acceptable to humans. As a result, humans can consume SP [37]. With regards to allergens, the WHO and International Union of Immunological Societies [38], quoted by Wu et al. [37], has not officially confirmed or recognized any allergens of SP (www.allergen.org). However, allergic reactions when SP was included in the diets were previously mentioned [37][39][40][41].
3. Application in Animal Feeding
The first mention of insects as animal feed dates to 1919, although their widespread usage began between 1960 and 1970 [16].
The protein with a higher biological value led to the opportunity of using SP in the livestock sector. Insects can only substitute the protein and fat typically added to animals’ diets. The ability to separately manipulate insect protein, different amino acids, and fat and add them in the right amounts to the feedstock might provide advantages to the feed sector. After extraction of oil, the cake remaining can be an alternative rich-protein, less expensive feedstuff for animal diets, especially for fish, poultry, and pigs [42][43], although, as Asimi et al. [44] described, only a minor part was used (about 25–30%).
The classical protein-rich feedstuffs for monogastric are soybean meal, characterized by a higher n-6 FA content; while SP contains a higher level of n-3 FA, close to linseed [9][10][45] and camelina [46]. Several researchers [47][48] have shown that the SP can potentially replace 50% of the classical protein source, respectively, fish meal or soybean meal (5–10% dietary inclusion).
According to Priyadharshini et al. [49] and Miah et al. [50], the dietary addition of SP did not affect the growth parameters or carcass traits of broiler and laying hens. However, the desired ratio of n-6 to n-3 is maintained if we speak in terms of meat quality. Although a complete replacement is typically possible, broilers are more susceptible to performance issues. In the same way, SP can adversely affect the capacity of growing quails to digest nutrients, primarily because of the presence of chitin and 1-DNJ [51].
Hens egg quality and yolk color can also be ameliorated using SP in the diet [49]. Although the important quantity of oil in SP can restrict the use of calves, the dietary addition of SP for cattle and monogastric has also been utilized mainly in Asian countries [43].
Priyadharshini et al. [49] obtained a higher deposition of fat and fur growth by including SP in rabbits’ feeds.
Additionally, fish meal was substituted entirely with SP in the fish diet by Shakoori et al. [52], with good results. The results showed that SP could stimulate the immune system in rainbow trout while inducing some anaemia-related symptoms.
SP oil reduced inflammation and oxidative stress in mice [28].
4. Environmental Effects
As was already indicated, one way to reduce the current issues arising from climate change and the environmental impact the world faces today is to employ insects for human and animal consumption. About 36% of global emissions of greenhouse gases (GHG) come from the agricultural sector, of which 78% are generated from cattle. The main GHG generated are methane (43%), nitrous oxide (29%), and carbon dioxide (27%) [16]. Depending on the species and production method, the obtaining of animal proteins has a significant negative influence on the environment. Animals generally require enormous land areas for breeding throughout their lives, as well as food resources (which impact the environment), water, fossil fuels, storage and packaging, etc.
Compared to livestock production, growing insects use less space and water and have a smaller negative impact on the environment and the economy. Insects are suggested as an alternative to reduce resource use. The edible fraction of insects is 80% to 90%. According to Ordoñez-Araque, [16], insects use 20 m2 to produce 1 kg of protein, 20 L water for 1 g of protein, 1 kg feed for 1 kg live weight, and 1 g average GHG/kg mass gain.
Fresh SP are a highly degradable by-product that pollutes the environment and gives off an unpleasant odour in the nearby locations. Large-scale pupae disposal can have significant environmental consequences in places that produce silk. According to Giacomin et al. [53], the mulberry is considered the starting point of the sericulture industry because it produces many of the leaves that silkworm larvae eat and has the capacity to sequester carbon. Culturing mulberry trees helps absorb a significant amount of harmful pollutants. Chemically derived fertilizers are not frequently used; 8.4 g of silk is equivalent to one mulberry tree. Mulberry fields reduce CO2 equivalent at about 735 times the weight of synthetic silk fiber per cultivated area.
Based on the hypothesis that adding fat-rich ingredients to the diet can reduce the amount of CH4 produced, we can assume that the SP could mitigate the main GHG. However, there is no evidence in the literature that nutritional strategies are more effective in decreasing the enteric CH4 level [11]. On the other hand, it was specified that PUFA inhibits the population of rumen protozoa that produce H2. It is well known that high H2 concentrations, produced by fermentation processes, cause a low redox potential and enhance propionate synthesis, which increases in the oil-supplemented groups [54]. About 9–25% of the rumen methanogens are associated with protozoa. Decreasing methane emissions is influenced by rumen protozoa [55]. Eliminating protozoa from the rumen (defaunation) has been shown to reduce methane emissions by 9–37% [55].
According to Sheikh et al. [43] and Thirumalaisamy et al. [54], one of the less expensive options for methane mitigation may be SP; this non-conventional oil source is utilized for human consumption in some countries. A better strategy to avoid waste and minimize the environmental impact of the silk industry also consists of using these valuable by-products to feed animals and poultry. In their study, Thirumalaisamy et al. [54] mentioned that sheep’s daily methane emissions were reduced by 23–26% over time by supplementing SP oil (2% of DM intake) on a daily or bimonthly basis.
Another strategy for sustainable development is to redirect agricultural wastes from pollution-producing applications and instead use them to make biofuels [36].
This entry is adapted from the peer-reviewed paper 10.3390/insects14030254
References
- Scrimgeour, C.; Gao, Y.; Oh, W.Y.; Shahidi, F. Edible Oil and Fat Products: Chemistry, Properties, and Safety Aspects. Chemistry of fatty acids. Chemistry of Fatty Acids. In Bailey’s Industrial Oil and Fat Products, 7th ed.; Shahidi, F., Ed.; Wiley Online Library: Hoboken, NJ, USA, 2005; pp. 1–40.
- Calder, P.C. Mechanisms of Action of (n-3) Fatty Acids. J. Nutr. 2012, 142, 592S–599S.
- Ohwada, T.; Yokokawa, T.; Kanno, Y.; Hotsuki, Y.; Sakamoto, T.; Watanabe, K.; Nakazato, K.; Takeishi, Y. Vascular composition data supporting the role of N-3 polyunsaturated fatty acids in the prevention of cardiovascular disease events. Data Brief 2016, 7, 1237–1247.
- Bartkiene, E.; Bartkevics, V.; Berzina, Z.; Klementaviciute, J.; Sidlauskiene, S.; Isariene, A.; Zeimiene, V.; Lele, V.; Mozuriene, E. Fatty acid profile and safety aspects of the edible oil prepared by artisans at small-scale agricultural companies. Food Sci. Nutr. 2021, 9, 5402–5414.
- Kumar, R.V.; Srivastava, D.; Kumar, U.; Kumar, M.; Singh, P. Bioprospecting of omega-3 fatty acid from silkworm pupal oil: From molecular mechanism to biological activities. J. Biol. Act. Prod. Nat. 2021, 10, 495–506.
- Orsavova, J.; Misurcova, L.; Ambrozova, J.V.; Vicha, R.; Mlcek, J. Fatty Acids Composition of Vegetable Oils and Its Contribution to Dietary Energy Intake and Dependence of Cardiovascular Mortality on Dietary Intake of Fatty Acids. Int. J. Mol. Sci. 2015, 16, 12871–12890.
- Vasseghian, Y.; Moradi, M.; Dragoi, E.-N.; Khaneghah, A.M. A review on mycotoxins detection techniques in edible oils. Int. J. Environ. Anal. Chem. 2020, 102, 2125–2139.
- Hăbeanu, M.; Hebean, V.; Taranu, I.; Ropota, M.; Lefter, N.A.; Marin, D. Dietary Ecologic Camelina Oil—A Beneficial Source of N-3 PUFA In Muscle Tissue and Health Status in Finishing Pig. Rom. Biotechnol. Lett. 2011, 16, 6564–6571.
- Hăbeanu, M.; Lefter, N.A.; Gheorghe, A.; Nagy, A.; Marin, D.; Ropotă, M. Effects of dietary flaxseed oil on the muscle fatty acid composition in Mangalitsa pigs in an extensive rearing system. S. Afr. J. Sci. 2014, 44, 240–244.
- Hăbeanu, M.; Lefter, N.A.; Gheorghe, A.; Untea, A.; Ropotă, M.; Grigore, D.-M.; Varzaru, I.; Toma, S.M. Evaluation of Performance, Nitrogen Metabolism and Tissue Composition in Barrows Fed an n-3 PUFA-Rich Diet. Animals 2019, 9, 234.
- Hăbeanu, M.; Lefter, N.A.; Gheorghe, A.; Ropota, M.; Toma, S.M.; Pistol, G.C.; Surdu, I.; Dumitru, M. Alterations in essential fatty acids, immunoglobulins (IgA, IgG and IgM), and enteric methane emission in primiparous sows fed hemp seed oil and their offspring response. Vet. Sci. 2022, 9, 352.
- Frančáková, H.; Ivanišová, E.; Dráb, Š.; Krajčovič, T.; Tokár, M.; Mareček, J.; Musilová, J. Composition of Fatty Acids in Selected Vegetable Oils. Potravin. Slovak J. Food Sci. 2015, 9, 538–542.
- Zhou, Y.; Zhao, W.; Lai, Y.; Zhang, B.; Zhang, D. Edible Plant Oil: Global Status, Health Issues, and Perspectives. Front. Plant Sci. 2020, 11, 1315.
- Longvah, T.; Mangthya, K.; Ramulu, P. Nutrient composition and protein quality evaluation of eri silkworm (Samia ricinii) prepupae and pupae. Food Chem. 2011, 128, 400–403.
- Mokaya, H.O.; Ndunda, R.M.; Kegode, T.M.; Koech, S.J.; Tanga, C.M.; Subramanian, S.; Ngok, B. Silkmoth pupae: Potential and less exploited alternative source of nutrients and natural antioxidants. J. Insects Food Feed 2022, in press.
- Ordoñez-Araque, R.; Quishpillo-Miranda, N.; Ramos-Guerrero, L. Edible Insects for Humans and Animals: Nutritional Composition and an Option for Mitigating Environmental Damage. Insects 2022, 13, 944.
- Suzuki, N.; Sawada, K.; Takahashi, I.; Matsuda, M.; Fukui, S.; Tokuyasu, H.; Shimizu, H.; Yokoyama, J.; Akaike, A.; Nakaji, S. Association between polyunsaturated fatty acid and reactive oxygen species production of neutrophils in the general population. Nutrients 2020, 12, 3222.
- Petermann, A.B.; Reyna-Jeldes, M.; Ortega, L.; Coddou, C.; Yévenes, G.E. Roles of the Unsaturated Fatty Acid Docosahexaenoic Acid in the Central Nervous System: Molecular and Cellular Insights. Int. J. Mol. Sci. 2022, 23, 5390.
- Zhou, Y.; Zhou, S.; Duan, H.; Wang, J.; Yan, W. Silkworm Pupae: A Functional Food with Health Benefits for Humans. Foods 2022, 11, 1594.
- Bourre, J.M.; Bonneil, M.; Clément, M.; Dumont, O.; Durand, G.; Lafont, H.; Nalbone, G.; Piciotti, M. Function of dietary polyunsaturated fatty acids in the nervous system. Prostaglandins Leukot. Essent. Fat. Acids 1993, 48, 5–15.
- Williams, C.M. Dietary fatty acids and human health. Anim. Res. 2000, 49, 165–180.
- Alessandri, J.M.; Guesneta, P.; Vancassel, S.; Astorg, P.; Denisa, I.; Langelier, B.; Aïd, S.; Poumès-Ballihaut, C.; Champeil-Potokar, G.; Laviall, M. Polyunsaturated fatty acids in the central nervous system: Evolution of concepts and nutritional implications throughout life. Reprod. Nutr. Dev. 2004, 44, 509–538.
- Rangacharyulu, P.V.; Giri, S.S.; Paul, B.N.; Yashoda, K.P.; Rao, R.J.; Mahendrakar, N.S.; Mohanty, S.N.; Mukhopadhyay, P.K. Utilization of fermented silkworm pupae silage in feed for carps. Bioresour. Technol. 2003, 86, 29–32.
- Kim, Y.J.; Lee, K.P.; Lee, D.Y.; Kim, Y.T.; Baek, S.; Yoon, M.S. Inhibitory effect of modified silkworm pupae oil in PDGF-BB-induced proliferation and migration of vascular smooth muscle cells. Food Sci. Biotechnol. 2020, 29, 1091–1099.
- Saviane, A.; Tassoni, L.; Naviglio, D.; Lupi, D.; Savoldelli, S.; Bianchi, G.; Cortellino, G.; Bondioli, P.; Folegatti, L.; Casartelli, M.; et al. Mechanical Processing of Hermetia illucens Larvae and Bombyx mori Pupae Produces Oils with Antimicrobial Activity. Animals 2021, 11, 783.
- Ryu, S.P. Silkworm pupae powder ingestion increases fat metabolism in swim-trained rats. J. Exerc. Nutr. Biochem. 2014, 18, 141–149.
- Long, X.; Song, J.; Zhao, X.; Zhang, Y.; Wang, H.; Liu, X.; Suo, H. Silkworm pupa oil attenuates acetaminophen-induced acute liver injury by inhibiting oxidative stress-mediated NF-κB signaling. Food Sci. Nutr. 2020, 8, 237–245.
- Long, X.; Zhao, X.; Wang, W.; Zhang, Y.; Wang, H.; Liu, X.; Suo, H. Protective effect of silkworm pupa oil on hydrochloric acid/ethanol-induced gastric ulcers. J. Sci. Food Agric. 2019, 99, 2974–2986.
- Wattanathorn, J.; Muchimapura, S.; Boosel, A.; Kongpa, S.; Kaewrueng, W.; Tong-Un, T.; Wannanon, P.; Thukhammee, W. Silkworm pupae protect against Alzheimer’s disease. Am. J. Agric. Biol. Sci. 2012, 7, 330–336.
- Baek, S.Y.; Li, F.Y.; Kim, J.H.; Ahn, C.; Kim, H.J.; Kim, M.R. Protein hydrolysate of silkworm pupa prevents memory impairment induced by oxidative stress in scopolamine-induced mice via modulating the cholinergic nervous system and antioxidant defense system. Prev. Nutr. Food Sci. 2020, 25, 389–399.
- Manosroi, A.; Boonpisuttinant, K.; Winitchai, S.; Manosroi, W.; Manosroi, J. Free radical scavenging and tyrosinase inhibition activity of oils and sericin extracted from Thai native silkworms (Bombyx mori). Pharm. Biol. 2010, 48, 855–860.
- Singh, K.P.; Jayasomu, R.S. Bombyx mori—A Review of its Potential as a Medicinal Insect. Pharm. Biol. 2002, 40, 28–32.
- Majumder, U.K.; Sengupta, A. Triglyceride composition of chrysalis oil, an insect lipid. J. Am. Oil Chem. Soc. 1997, 56, 620–623.
- Yoon, S.; Wong, A.K.N.; Chae, M.; Auh, J.-H. Comparative Characterization of Protein Hydrolysates from Three Edible Insects: Mealworm Larvae, Adult Crickets, and Silkworm Pupae. Foods 2019, 8, 563.
- Deori, M.; Boruah, D.C.; Devi, D.; Devi, R. Antioxidant and antigenotoxic effects of pupae of the muga silkworm Antheraea assamensis. Food Biosci. 2014, 5, 108–114.
- Łochynska, M.; Frankowski, J. The biogas production potential from silkworm waste. Waste Manag. 2018, 79, 564–570.
- Wu, X.; He, K.; Cirkovic Velickovic, T.; Liu, Z. Nutritional, functional, and allergenic properties of silkworm pupae. Food Sci. Nutr. 2021, 9, 4655–4665.
- World Health Organization (WHO). Healthy Diet: Fact Sheet. 2015. Available online: http://www.who.int/nutrition/publications/nutrientrequirements/healthydiet_factsheet394.pdf?ua¼1 (accessed on 15 January 2023).
- Ji, K.M.; Zhan, Z.K.; Chen, J.J.; Liu, Z.G. Anaphylactic shock caused by silkworm pupa consumption in China. Allergy 2008, 63, 1407–1408.
- Choi, G.S.; Shin, Y.S.; Kim, J.E.; Ye, Y.M.; Park, H.S. Five cases of food allergy to vegetable worm (Cordyceps sinensis) showing cross-reactivity with silkworm pupae. Allergy 2010, 65, 1196–1197.
- Feng, Y.; Chen, X.M.; Zhao, M.; He, Z.; Sun, L.; Wang, C.Y.; Ding, D.F. Edible insects in China: Utilization and prospects. Insect Sci. 2018, 25, 184–198.
- Herman, R.A.; Yan, C.-H.; Wang, J.-Z.; Xun, X.-M.; Wu, C.-K.; Li, Z.-N.; Ayepa, E.; You, S.; Gong, L.-C.; Wang, J. Insight into the silkworm pupae: Modification technologies and functionality of the protein and lipids. Trends Food Sci. Tech. 2022, 129, 408–420.
- Sheikh, I.U.; Banday, M.T.; Baba, I.A.; Adil, S.; Shaista, S.N.; Bushra, Z.; BulbuI, K.H. Utilization of silkworm pupae meal as an alternative source of protein in the diet of livestock and poultry: A review. J. Entomol. Zool. Stud. 2018, 6, 1010–1016.
- Asimi, O.A.; Bhat, T.H.; Nasir, H.; Irfan, K. Alternative Source of Protein “Silkworm Pupae” (Bombyx mori) In Coldwater Aquaculture. Int. J. Poult. Sci. 2017, 1, 1–4.
- Matthäus, B.; Özcan, M.M. Fatty Acid Composition, Tocopherol and Sterol Contents in Linseed (Linum usitatissimum L.) Varieties. Iran. J. Chem. Chem. Eng. 2017, 36, 147–152.
- Ostrikov, A.N.; Kleimenova, N.L.; Kopylov, M.V.; Bolgova, I.N. The study of the fatty acid composition of camelina oil obtained by cold pressing. IOP Publ. Conf. Ser. Earth Environ. Sci. 2021, 640, 042009.
- Valerie, H.; Tran, G.; Giger-Reverdin, S.; Lebas, F.; Silkworm Pupae Meal. Feedipedia, a Programme by INRA, CIRAD, AFZ and FAO. 2015. Available online: http://www.feedipedia.org/node/199 (accessed on 12 December 2022).
- Fagoonee, I. Possible growth factors for chickens in silkworm pupae meal. Brit. Poult. Sci. 1983, 24, 295–300.
- Priyadharshini, P.; Joncy, M.A.; Saratha, M. Industrial utilization of silkworm pupae—A review. J. Int. Acad. Res. Multidiscip. 2017, 5, 62–70.
- Miah, M.; Singh, Y.; Cullere, M.; Tenti, S.; Zotte, A.D. Effect of dietary supplementation with full-fat silkworm (Bombyx mori L.) chrysalis meal on growth performance and meat quality of Rhode Island Red Fayoumi crossbred chickens. Ital. J. Anim. Sci. 2020, 19, 447–456.
- Zotte, A.D.; Singh, Y.; Squartini, A.; Stevanato, P.; Cappellozza, S.; Kovitvadhi, A.; Subaneg, S.; Bertelli, D.; Cullere, M. Effect of a dietary inclusion of full-fat or defatted silkworm pupa meal on the nutrient digestibility and faecal microbiome of fattening quails. Animal 2021, 15, 100112.
- Shakoori, M.; Gholipour, M.; Naseri, S. Effect of replacing dietary fish meal with silkworm (Bombyx mori) pupae on hematological parameters of rainbow trout Oncorhynchus mykiss. Comp. Clin. Path. 2014, 24, 139–143.
- Giacomin, A.M.; Garcia, J.B., Jr.; Zonatti, W.F.; Silva-Santos, M.C.; Laktim, M.C.; Baruque-Ramos, J. Silk industy and carbon footprint mitigation. IOP Publ. Conf. Ser. Mater. Sci. Eng. 2017, 254, 192008.
- Thirumalaisamy, G.; Malik, P.K.; Trivedi, S.; Kolte, A.P.; Dhali, A.; Bhatta, R. Effect of silkworm (Bombyx mori) pupae oil supplementation on enteric methane emission and methanogens diversity in sheep. Anim. Biotechnol. 2022, 33, 128–140.
- Belanche, A.; de la Fuente, G.; Charles, J.N. Study of methanogen communities associated with different rumen protozoal populations. FEMS Microbiol. Ecol. 2014, 90, 663–677.
This entry is offline, you can click here to edit this entry!
