Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Gastroenterology & Hepatology
Crohn’s disease (CD) is a chronic immune mediated disorder that most commonly affects the small bowel and/or the large bowel. Treatment targets in CD include mucosal healing assessed via ileocolonoscopy and transmural healing assessed through cross-sectional imaging modalities such as magnetic resonance enterography (MRE).
- magnetic resonance enterography
- Crohn’s disease
- diagnostics
- endoscopy
1. Introduction
Crohn’s Disease (CD) is a chronic inflammatory condition with an incident rate of over 1 in 300 in developed countries [1]. Though CD can affect the entire gastrointestinal tract, approximately 70% of patients will have small intestinal involvement, with endoscopic manifestations including ulceration and inflammation, which can progress to penetrating and/or stricturing complications when untreated [2,3,4,5]. Advances in medical therapy and the availability of multiple different classes of agents have culminated in more stringent treatment targets plus the need for regular objective assessments of disease activity to ensure optimal and durable outcomes for patients.
The gold standard for CD activity assessment remains ileocolonoscopic examination [6], with established definitions for endoscopic remission such as a Simplified Endoscopy Score in CD (SES-CD) of ≤2 points or Crohn’s Disease Endoscopic Index of Severity (CDEIS) <3 and a lack of ulceration [7,8]. However, ileocolonoscopy has several limitations that preclude regular and repeated endoscopic assessment, including cost and resource constraints, the need for bowel preparation and sedation anaesthesia, plus procedural risks including bowel perforation and bleeding. Additionally, assessment of the small bowel is limited to the terminal ileal mucosa with a standard ileocolonoscopy, yet the proximal small bowel and deeper submucosal layers cannot be evaluated [9].
Recent advances in the quality and accuracy of non-invasive imaging modalities have resulted in these measures being preferentially used as surrogates for endoscopic assessment, and guidelines support the use of magnetic resonance enterography (MRE), intestinal ultrasound (IUS), and computed tomography enterography (CTE) as adjuncts to ileocolonoscopy [7]. The key goal of any non-invasive method of CD activity detection is to achieve equivalent sensitivity to that of an ileocolonoscopy, including mucosal activity. Indeed, the advantages of non-invasive MRE, including the provision of transmural and extra-mural information, convenience, and improving access and cost versus the known disadvantages of endoscopy, raise the question as to whether MRE could even supersede ileocolonoscopy as the first-line investigation in small bowel CD.
2. MRE Parameters That Correlate with Disease Activity
2.1. Bowel Wall Thickness
Bowel wall thickness (BWT) is a shared parameter across the cross-sectional imaging modalities and is validated as a marker of CD activity (see Figure 1, Figure 2 and Figure 3) [10]. Recent consensus statements depict a BWT > 3 mm as abnormal in either the terminal ileum or colon [11,12]. As an individual parameter, BWT has been shown to be an independent predictor of active mucosal disease; however, increased BWT may also reflect fibrostenosing CD [13,14]. Within mucosally active CD, multiple studies have shown the presence of ulcers endoscopically to be a critical marker of severity, with ulcerated segments more likely to demonstrate increased overall BWT on MRE [10,15,16,17]. In a prospective study of 48 patients with CD undergoing paired MRE and colonoscopy for disease activity assessment, a significant difference in BWT between non-ulcerated and ulcerated endoscopic segments (4.29 ± 1.46 mm vs. 5.61 ± 1.26 mm, p < 0.001) was observed. Moreover, a further significant reduction in mean BWT was seen in endoscopically normal mucosa and non-ulcerated inflammation (2.83 ± 0.61 mm vs. 4.29 ± 1.46 mm, p < 0.001) [18]. In the absence of ulceration on endoscopy, increased BWT as a parameter to assess CD activity appears to be less sensitive and specific; thus, additional parameters should be considered. In two subsequent studies by the same authors, the prevalence of a BWT >3 mm was only seen in 46 to 74% of patients with mildly active CD on endoscopy [16,19]. Other studies have reported similar findings, with a BWT >3 mm having a sensitivity of only 35% in detecting mucosally active CD in the absence of ulcers or erosions on endoscopic examination [20]. Finally, as an individual parameter, there is good reliability in detecting increased BWT when assessed by experienced radiologists, with Jairath et al. reporting an inter-class correlation of 0.72 (95% confidence interval (CI), (0.62–0.80)) when assessing BWT within the terminal ileum [21].
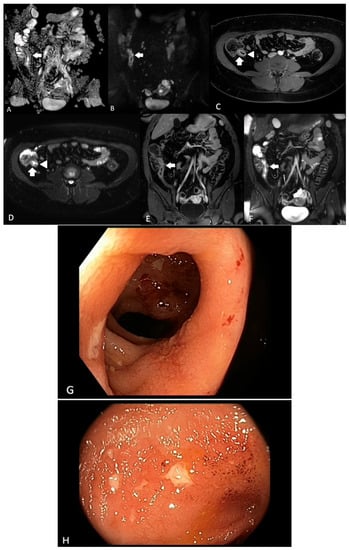
Figure 1. A 31-year-old female with known ileal CD. (A) ADC map and (B) DWI sequences demonstrating terminal ileal wall diffusion restriction indicating active inflammation (arrow); (C) axial T2-weighted fat-saturated sequence demonstrating terminal ileal wall thickening and intra mural oedema (arrow) with ulceration medially (arrowhead); (D) axial T2-weighted fat-saturated sequence demonstrating terminal ileal wall thickening and intra mural oedema (arrow) with ulceration medially (arrowhead); (E) coronal T1-weighted fat-saturated post-contrast sequence demonstrating terminal ileal wall thickening and hyperenhancement (arrow); (F) coronal T2-weighted fat-saturated sequence demonstrating terminal ileal wall thickening and intra mural oedema (arrow), (G) and (H). Corresponding ileal endoscopic image (SES-CD = 5).
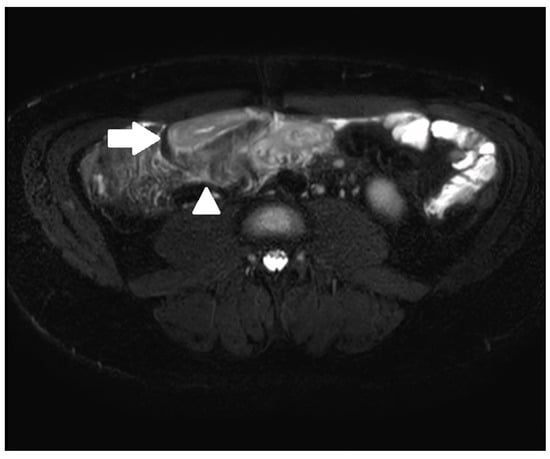
Figure 2. A 33-year-old male with ileal CD. Axial T2-weighted fat-saturated sequence demonstrating terminal ileal wall thickening and intra mural oedema (arrow) with peri enteric oedema (arrowhead).
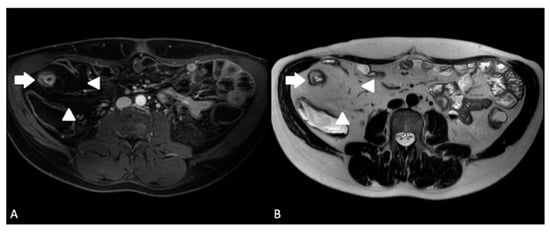
Figure 3. A 24-year-old male with ileal CD. (A) Axial T1-weighted fat-saturated post-contrast sequence demonstrating terminal ileal wall thickening and hyperenhancement (arrow) with creeping fat (arrowheads); (B) axial T2-weighted sequence demonstrating terminal ileal wall thickening and intra mural oedema (arrow) with creeping fat (arrowheads).
2.2. Bowel Wall Contrast Enhancement
Bowel wall contrast enhancement can aid the differentiation of active luminal CD from fibrostenosing changes. Key findings in post-contrast MRE sequences include the degree and pattern of bowel wall enhancement [11,12]. Patterns of bowel wall enhancement are categorised as homogenous, mucosal, or layered stratification (see Figure 1, Figure 3 and Figure 4). Mucosal pattern enhancement refers to the increased enhancement of the mucosal layer associated with increased BWT. Layered enhancement is defined as the enhancement of the mucosa (inner layer) and serosa (outer layer) with sparing of the middle layer (representing submucosa and muscular layer) [22]. Multiple studies have demonstrated that both mucosal and layered enhancement patterns are associated with histologically active disease [22,23,24,25]. For instance, in a study of 53 CD patients undergoing bowel resection, a preoperative MRE assessment of CD activity demonstrated that both the degree of bowel wall enhancement (mild vs. marked) and the pattern of enhancement (layered vs. homogenous) on delayed T1 post-contrast sequences were altered significantly between different histopathological grades of inflammation assessed on surgical resection specimens [13]. In a subsequent study using ileocolonoscopy as a reference standard, significant differences in bowel wall contrast enhancement were observed between normal mucosa and mucosally active CD [18].
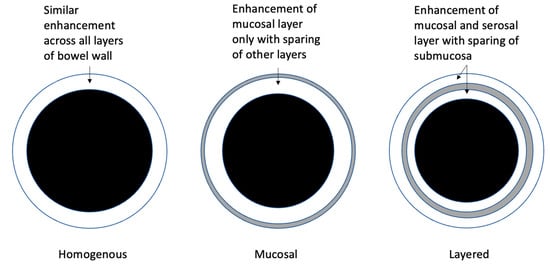
Figure 4. Categories of bowel wall enhancement.
2.3. T2 Mural Signal Intensity/Intramural Oedema
T2 mural signal intensity, also known as intramural oedema, is a finding unique to MRE and is not seen with other forms of cross-sectional imaging such as CTE. It is demonstrated by a high signal intensity within the submucosa of bowel wall in affected segments and is best appreciated on T2-weighted fat-suppressed images (see Figure 1 and Figure 3) [11]. Fat suppression allows for differentiation with chronic fibrotic CD, which also presents with a high signal on submucosal T2-weighted images [11]. Intramural oedema is known to correlate with degrees of active inflammation in CD seen via endoscopic assessment and on histology of surgical resection specimens [10,26]. In the absence of endoscopically active CD, oedema is seldom present [16,18]. In a study of 50 patients undergoing paired MRE and colonoscopy, intramural oedema was present in only 5/198 (2.5%) of endoscopically normal bowel segments [16]. Studies have demonstrated that mural signal intensity is able to differentiate grades of endoscopic disease when small bowel ulceration is present; however, it is less sensitive in non-ulcerative, endoscopically active CD [10,18]. In active, ulcerative CD, its prevalence is significantly higher, with studies reporting a sensitivity ranging between 68–84% [16,19,20]. Moreover, intramural oedema demonstrates good reliability, with one study demonstrating an inter-class correlation of 0.80 (95% CI, (0.69–0.89)) within the terminal ileum, with lower reliability in colonic segments [21].
2.4. Ulceration
The finding of ulceration on MRE is known to be correlated with active inflammation seen in CD through endoscopic examination and histology [10,26]. On MRE, superficial and deep ulcers can be identified on T2 and post-contrast T1 imaging as areas of deep depressions in the mucosal surface of a thickened segment (see Figure 1) [10,11]. With advanced CD, a cobblestone appearance on MRE can represent areas of deep ulceration alternating with thickened mucosal folds [27]. As expected, in the absence of endoscopically active CD, ulceration is seldom appreciable on MRE, with one study demonstrating a 1.5% false positive rate [16]. Studies have demonstrated the capacity of ulceration on MRE to differentiate between grades of endoscopic CD though its prevalence in MRE, with non-ulcerative endoscopically active CD being reportedly as low as 9% [10]. Endoscopically active CD with visible ulceration is more likely to portend ulceration on MRE, with studies reporting a sensitivity of 53–78% [16,19,20]. Ulceration on MRE has a fair reliability, with one study demonstrating an interclass correlation coefficient of 0.60 (0.45–0.72) within the terminal ileum but reduced reliability in colonic segments [21].
2.5. Diffusion-Weighted Imaging
Diffusion-weighted imaging (DWI) is a specialised sequence in MRE. DWI reflects water motility (diffusion) within tissue, with pathological alterations resulting in signal changes (i.e., restricted diffusion) (see Figure 1) [28]. DWI is performed by utilising T2-weighted fat-suppressed sequences and adding a diffusion gradient. The gradient is quantified by a coefficient (b-value) that is used to construct a corresponding apparent diffusion coefficient (ADC) map. A meta-analysis demonstrated that DWI-MRE had a pooled sensitivity and specificity of 93% and 91% in detecting small bowel CD compared to a reference standard including ileocolonoscopy, histopathology, and alternative radiological methods [29]. However, a lack of blinding to the reference standard may reflect bias in these studies and an overestimation of accuracy [29]. When blinded to the reference standard, DWI-MRE sensitivity and specificity in detecting CD was reduced to 79% and 61%, respectively [29]. Qualitative and quantitative assessment of DWI and ADC map signals have been described in the literature. However, the heterogeneity of imaging techniques and studies have limited the use of ADC map thresholds for the detection of active inflammation and the distinction between inflammation and fibrosis. [28].
2.6. Stenosis and Pre-Stenotic Dilatation
CD-related stenosis is represented cross-sectionally by a segment of luminal narrowing of 50% compared to the adjacent bowel loop, and in the case of a definite stricture, it is further confirmed by pre-stenotic luminal dilatation (≥3 cm). A ‘probable stricture’ is defined by a luminal narrowing of 50% compared to the adjacent bowel loop without pre-stenotic dilatation (<3 cm) [11]. Specific MRE findings suggestive of an active inflammatory stricture include bowel wall contrast enhancement, BWT, and bowel wall oedema in the affected segment of stenosis [30]. These changes are best appreciated on post-contrast T1 and fat-suppressed T2 image sequences. In addition, the assessment of small intestine motility on cine sequences allows for a differentiation between fixed stenotic lesions and temporary luminal narrowing. When cine sequences are not available, reviewing the site of a possible stricture on different sequences may also provide information as to whether this site remains narrowed or not, given the different sequences are obtained over different timepoints. Regarding fibrotic stenosis/strictures, these are thin-walled and appear hypointense on T1 and T2 sequences and often occur in conjunction with submucosal fibrofatty proliferation. However, as previously mentioned, overlap between inflammatory and fibrotic appearances of strictures is frequently encountered; thus, it is often not possible to differentiate between the two processes [31]. Initial studies demonstrated that MRE had a poor sensitivity in detecting stenosis compared to balloon enteroscopy in small bowel CD, with a reported sensitivity of 40.8% (95% CI, 0.31–0.49) [32]. However, when compared to surgical histology, a recent systematic review reported that MRE detected CD-related strictures with a sensitivity between 75% and 100% and a specificity between 91% and 96% [33].
2.7. Motility Sequence Assessment/Cine Sequences
Reduced intestinal motility is well described in patients with active CD, with cine sequences allowing for the assessment of intestinal motility [34]. More recently, an automated software algorithm was developed, facilitating the calculation of the motility index (MI) [35]. Menys et al. demonstrated that in patients with active CD, a reduction in MI was negatively correlated with histologically (r = −0.61; (0.7, −0.5)) and endoscopically active CD (r = −0.59; (0.7, −0.4)) [36]. In a subsequent study by Dreja et al., the MI was also inversely correlated with mural thickness in the terminal ileum, with a lower MI in patients with active CD versus controls in the ileum [37]. Hence, intestinal motility not only appears to be reduced in patients with active CD, but there is a potential for the quantification of motility by implementing the MI as an adjunctive parameter in MRE assessment. Further studies are required to determine whether normalisation of the MI may be a useful treatment target in patients with CD.
2.8. Fibrofatty Proliferation/Creeping Fat
Fibrofatty proliferation presents as fat wrapping around an inflamed bowel loop. On MRE, this presents as reduced signal intensity on T1-weighted images (see Figure 3) [11]. The presence of fibrofatty proliferation is pathognomonic for CD and is suggestive of longstanding disease [38]. Studies have demonstrated that creeping fat may indeed persist despite endoscopic response to therapy [17,39]. In a prospective study of 28 patients receiving anti-TNF therapy for CD, creeping fat did not significantly differ between baseline and repeat MRE at 52 weeks [39]. Moreover, the presence of creeping fat was not associated with disease relapse [39]. Therefore, it may be assumed that the presence of creeping fat is an established MRE parameter that is not affected by endoscopic or mucosal disease activity and is suggestive of chronic CD. Fibrofatty proliferation on MRE has a fair inter-observer reliability, with one study demonstrating an interclass correlation coefficient of 0.47 [21].
2.9. Mesenteric Vascularity (Comb Sign)
The comb sign is an MRE parameter associated with increased CD activity and occurs as a result of vascular engorgement of the vasa recta that extend perpendicularly from the mesentery to the inflamed bowel wall. The comb sign often occurs in parallel with increased stranding of mesenteric fat, and in conjunction, they are known to correlate with extensive and active CD [40].
2.10. Inflammatory Lymph Nodes
Inflammatory lymph nodes also often occur in conjunction with increased mesenteric vascularity. Measurements of >15 mm in the short axis are considered pathological and may be indicative of active CD [12]. The presence of pathological lymph nodes may be significant in differentiating between endoscopic activity and remission. Rimola et al. demonstrated that CD segments associated with active endoscopic disease were more likely to demonstrate pathological regional lymphadenopathy in the adjacent mesentery [18]. Though more likely to be associated with endoscopically active CD, as an individual parameter, the presence of lymph nodes was poorly sensitive, with only 17% of non-ulcerated inflamed bowel segments having associated pathological lymphadenopathy [18]. When compared with histopathological resection specimens, Zappa et al. were unable to demonstrate the ability of lymph nodes to distinguish between mild and severe grades of inflammation [13]. Thus, in the absence of other parameters of disease, the presence of pathological lymph nodes cannot be utilised to denote the likelihood of endoscopically active disease via MRE.
2.11. Abscess Detection
Abscesses are a known complication of advanced penetrating CD. On MRE, they are characterised by rim enhancement on post-contrast T1-weighted images and a high central signal on T2-weighted sequences [11]. Abscesses are frequently surrounded by fat stranding. In a previous meta-analysis, the pooled sensitivity of MRI for the detection of abscesses was 86%, with a specificity of 93% when compared to histopathology as the reference standard [41].
2.12. Enteric Fistula
Enteric fistulas are almost always seen proximal to a region of bowel stenosis. On cross-sectional imaging, enteric fistulas can be seen as a connection between bowel segments, intrabdominal organs, and/or skin. Fistula tracts are best appreciated on T1-weighted post-contrast sequences and demonstrate tract wall enhancement. In a meta-analysis, the pooled sensitivity of MRE for the diagnosis of fistulas was 76%, and the specificity was 96% when compared to histopathology specimens as the reference standard [41].
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics13061061
This entry is offline, you can click here to edit this entry!
