Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Coral reefs are vital ecosystems with high biodiversity and ecological services for coastal communities. Climate change is accelerating, with detrimental consequences on coral reefs and related communities.
- coral
- Climate change
- ocean
1. Introduction
Scleractinians, or stony corals, emerged during the Cambrian period and constructed the earliest reefs, dating to approximately 410 million years ago [1][2][3]. Five major coral extinctions have occurred since then, all of which have been linked to rising temperatures and higher levels of carbon dioxide in the atmosphere [2][4].
While coral reef research commenced more than 100 years ago, concern over the state of coral reefs is relatively recent, occurring only in the last four decades. In 1981, at the 4th International Coral Reef Symposium, Edgardo Gomez initiated the conversation on threats to coral reefs by presenting his concerns to the scientific community [5][6]. Coral reefs are considered an important marine resource for coastal communities, and the conference participants were mainly focused on coral reef management and environmental impacts and related fisheries activities [5][7][8]. In addition to the natural stresses that have always existed on coral reefs, such as storms, freshwater inundation, and seismic and volcanic events, there is growing evidence of new emerging threats potentially causing global damage to coral reefs [7][8][9][10].
At current extinction rates, it is estimated that we are commencing the sixth mass extinction event [11][12][13], with individual extinctions occurring approximately 1000-fold faster than the expected background extinction rate. Theoretically, species extinctions occur at a rate proportional to the rate of speciation or the creation of new species [14]. Current extinction rates are much higher than speciation rates. This is largely due to the fact of anthropogenic factors [15][16], such as habitat destruction [17], deforestation [18], pollution [7], ocean acidification, climate change resulting from greenhouse gas emissions, and overexploitation of ecological resources [19][20][21]. It is estimated that 75% of species will go extinct unless human pressures on the environment are scaled back soon [20][22][23][24][25]. Further management efforts are required to reduce the impacts of climate change and human anthropogenic stress towards coral reef communities [7].
Anthropogenic pressures on reefs have been the dominant factor damaging coral reefs through a range of stresses (Figure 1). Unsustainable land-based human activities, such as deforestation, poorly regulated agriculture, and urban/industrial development, are major contributors to the release of excessive sediments and nutrients into the environment [26][27][28]. Increases in human-caused greenhouse gas emissions are the primary factor in the current climatic shift around the world [29][30]. The ocean acts as a massive sink that absorbs carbon dioxide, resulting in the acidification of the oceans [31][32]. Coral bleaching and widespread damage to the coral reef ecosystem have become increasingly common because of thermal stress brought on by rising ocean temperatures [33]. Lack of food to sustain the coral reef ecosystem and disruption of larvae dispersal are both attributable to altered currents, upwelling, and/or vertical mixing brought on by changing currents and winds [34]. Storms and cyclones are “agents of mortality” on coral reefs and can have a direct impact on the structure and local distribution of coral reef assemblages, especially through the large waves they produce [35]. Reduced salinity caused by heavy rainfall and enhanced surface run-off onto nearshore reefs during cyclones, storms, and heavy precipitation rates can lead to algal blooms and other devastating results [36][37]. The rise in sea level, caused by thermal expansion and the melting of ice on land, has varied across different regions of the world over the past century, with an average increase of approximately 20 cm [38]. The sedimentary mechanisms triggered by the rising sea levels have the potential to intensify and jeopardize crucial physiological reef processes, such as photosynthesis, feeding, and recruitment, thereby posing a severe threat to coral reefs and related ecosystems, such as seagrass meadows and mangrove forests [39][40]. This threat, coupled with increasing carbon dioxide (CO2) emissions, can negatively impact these vital ecosystems. Without effective local measures and a concerted effort to reduce carbon dioxide emissions, these effects are projected to intensify, leading to an unprecedented degradation of marine biodiversity and ecological balance [41][42].
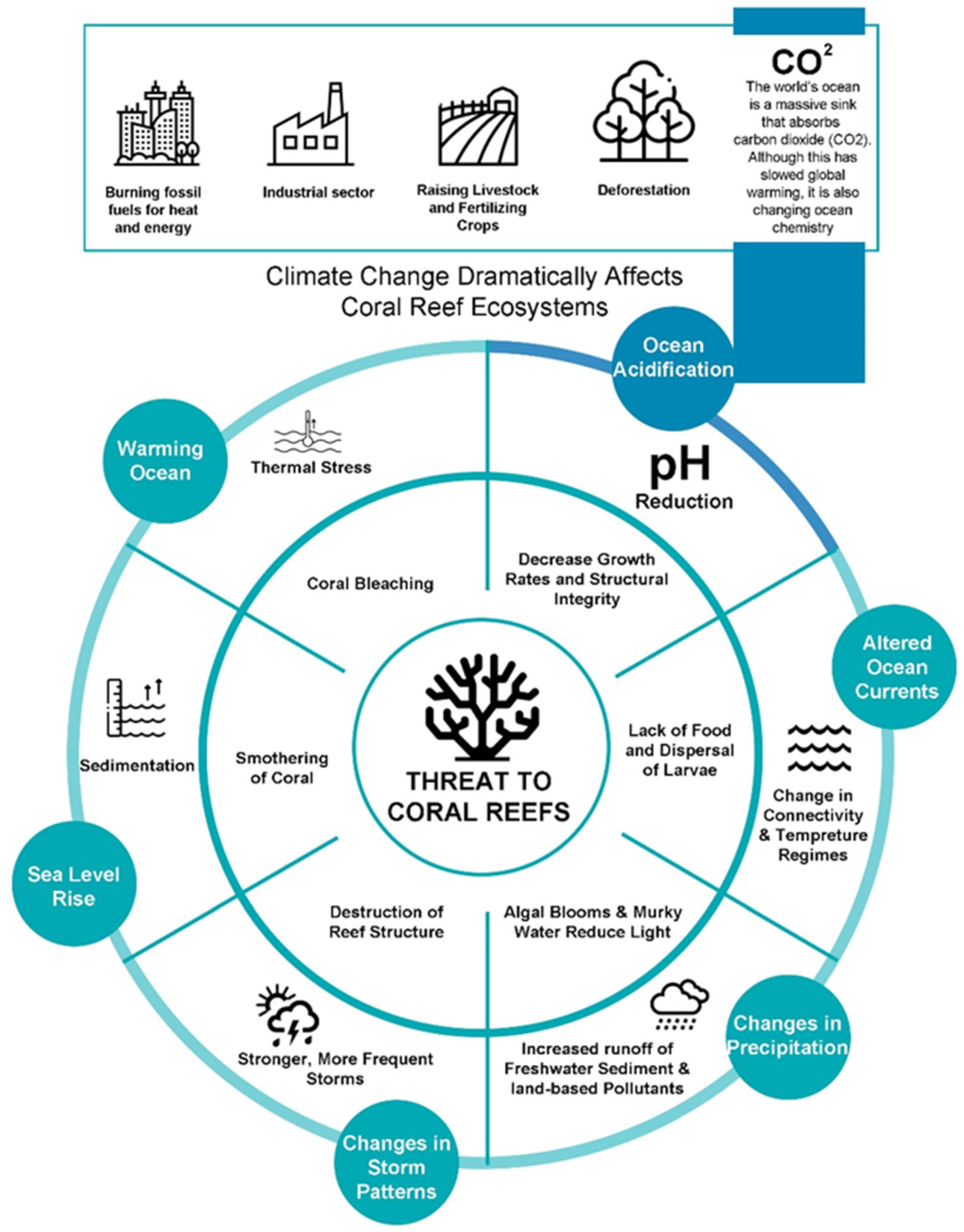
Figure 1. Threats to coral reefs posed by factors related to climate change.
2. The Evolution of Coral Reef under Changing Climate
Coral reefs are a vital marine ecosystem service, providing high biodiversity and supporting the livelihoods of coastal communities. However, ocean warming and temperature are the largest threats to corals from anthropogenic climate change [43]. Between 1997 and 2018, the global average percentage of coral cover was approximately 32%, but by 2100, RCP 8.5 predicts a global decline in coral cover of 5 and 15%, equating to a relative global decline of more than 40% [44][45]. This decline is due to the fact that sea surface temperatures (SSTs) are projected to increase by more than 3 °C by the turn of the century [46]. These declines could have significant ecological and socioeconomic impacts, particularly in coastal communities that rely on coral reefs for food, tourism, and other ecosystem services.
For example, The Republic of Palau, a small Micronesian nation, has already experienced significant losses in coral reef cover [47]. Over 87% of Palau’s households are linked to coral reef-associated activities, which are critical to the country’s economic and social well-being. While tourism, particularly ecotourism, is a significant contributor to GDP growth, tax revenue, and employment, climate change-related stressors have caused a steady decline in coral reef cover. This decline has indirectly caused a major decline in tourism, threatening the country’s economic sustainability [48]. According to Barnett [49], climate change is a significant threat to food security for people in Pacific SIDS, primarily due to the decline in fisheries output resulting from the impact of climate change on total coral cover.
Apart from impacting the socioecological structure, the impact of climate change can have cascading effects on the entire reef ecosystem, affecting the abundance and diversity of other marine species that depend on corals for food and shelter. Up to 14% of species may be in imminent danger of extinction at a warming of 1.5 °C and up to 29% at a warming of 3 °C. This rise in ocean temperature will probably force coral to colonize higher latitudes that currently lack reefs [50][51][52]. However, various factors, including the need for a suitable substrate [53], connectivity to other reefs [54], ocean acidification [55], and light intensity [56], may outweigh the advantages of reefs as they expand to high latitudes [45].
2.1. The Threat of Climate Change to Coral Reefs: Investigating the Impacts of Temperature and Ocean Acidification
Climate-induced changes in temperature are a major threat to coral reef ecosystems, and extensive research has highlighted several key areas for investigation [57][58], with marine heatwaves, solar radiation, heat tolerance, and thermal thresholds representing the most promising areas for future research. Marine heat waves have become increasingly prevalent and intense as a result of climate change. These extreme events, characterized by prolonged periods of elevated water temperatures, significantly impact coral reef ecosystems. For instance, the mass global coral bleaching event of 2016–2017 was the most extensive and long lasting on record, as documented by Eakin et al. [59]. The event, which was associated with the El Niño Southern Oscillation (ENSO), had varying impacts on coral reefs worldwide [60], with some regions experiencing more severe bleaching than others, as reported by Kim et al. [61].
Corals are thermophilic, but their thermal tolerance is narrowly defined [62][63]. For instance, the rate of calcification increases with temperature up to a threshold level, beyond which it declines [64][65][66]. Tropical corals live close to their upper thermal limits and are, therefore, highly sensitive to periods of elevated sea surface temperatures and ocean warming [67][68]. Coral reefs in the Persian Gulf have been observed to have the highest upper-temperature thresholds of approximately 35–36 °C [69]. However, it has also been noted that these corals remain highly vulnerable to thermal stress when temperatures surpass their local maximum summer temperatures [70]. The escalating frequency and gravity of thermally induced mass bleaching events have sparked worldwide attention to the elevated temperature impacts on corals [28]. As a result, research endeavors have focused on establishing maximum thermal tolerance thresholds and variations in diverse coral species and regions and exploring potential coral refugia to brace for future ocean warming [71].
Corals rely on their symbiotic relationship with unicellular algae of the genus Symbiodinium for photosynthesis, and over 90% of their energy budget is needed for essential functions, such as calcification, tissue growth, and reproduction [66]. This critical association is threatened when corals experience thermal stress, such as elevated sea surface temperatures (SST), resulting in coral bleaching, where the algal endosymbionts are expelled. The resulting impairment and expulsion of the algal symbionts are linked to reactive oxygen species (ROS) generation from the host, the algal symbiont, or both, triggering a host immune response [72].
Protracted coral bleaching can lead to extensive coral mortality, severely affecting the ecosystem and associated reef fauna. Based on the timeline cocitation analysis, it was evident that the Red Sea (Cluster #3) and Great Barrier Reef (GBR) (cluster #8) are major research hotspots in terms of geographic regions. Although the Persian Gulf is a hot sea that supports coral reef ecosystems, the Red Sea harbors corals with greater thermal stress tolerance, with some coral genotypes capable of surviving temperatures over 5 °C above their summer maxima [71][73]. Corals in the southern end of the Red Sea are more heat resistant, surviving prolonged high temperatures, while the northern Red Sea benefits from heat-resistant genotypes that have migrated from the south [74]. The importance of broad latitudinal temperature gradients in promoting adaptation to high temperatures and exchanging heat-resistant genotypes across latitudes for genetic rescue in coral reefs is exemplified in the evolutionary history of coral reefs in the northern Red Sea [9][71]. On the other hand, the GBR, known as the world’s largest coral ecosystem, was severely impacted by the 2015–2016 climate change-amplified strong El Niño event that triggered the warmest temperatures on record. This resulted in a massive bleaching event affecting nearly 90% of reefs along the northern region, leading to a loss of approximately 30% of live coral cover in the following six months [28][75][76]. Research has increasingly linked climate change to a rise in coral diseases. Bruno et al. [77] used a high-resolution satellite dataset to investigate the relationship between temperature anomalies and coral disease on a large spatial scale of 1500 km in Australia’s Great Barrier Reef. Their findings showed a significant positive correlation between warm temperature anomalies and the incidence of the white syndrome, an emergent disease in Pacific reef-building corals. In a similar vein, Tignat-Perrier et al. [78] noted a decline in populations of two gorgonian species (Paramuricea clavata and Eunicella cavolini) found in the Mediterranean Sea due to the fact of microbial diseases during thermal stress events. These studies illustrate the growing concern that climate change is contributing to the increased incidence and severity of coral diseases, which could ultimately lead to a decline in the health of marine ecosystems.
In the past, studies on the impact of climate change on coral reefs primarily centered on the thermal tolerance of corals and the consequences of massive, abrupt coral loss on organisms associated with reefs [79]. However, research has recently shifted towards investigating the distinct and synergistic effects of ocean warming and ocean acidification resulting from increased atmospheric CO2 levels. The timeline co-citation analysis reveals that these emerging research fields are highly significant with recent citation bursts, as evidenced by their identification as Cluster #2 (Ocean acidification) and Cluster #10 (Elevated CO2), respectively.
The escalation of atmospheric carbon dioxide (CO2) concentrations has resulted in ocean acidification, which is among the foremost threats to coral reef ecosystems. Forecasts for 2100 anticipate a rise in CO2 concentrations to between 540 and 970 ppm, leading to a global decrease in seawater pH by 0.14 to 0.35 units [31][80][81][82]. As demonstrated by Fabricius et al. [80], ecological traits of coral reefs will gradually transform as seawater pH decreases to 7.8, and a decline below this level (at 750 ppm pCO2) would be catastrophic for these ecosystems. Ocean acidification reduces the availability of carbonate ions that corals require to form their calcium carbonate skeletons, ultimately leading to a decrease in coral calcification rates [33]. Ocean acidification has also been shown to decrease the ability of coral larvae to settle and survive [83] and increase their susceptibility to disease [84]. Research has shown that even modest increases in ocean acidity can impact the physiological processes of corals. For example, exposure to high levels of CO2 reduces coral growth and calcification rates [80][83]. In addition to the direct effects on coral physiology, ocean acidification can have cascading impacts on the entire coral reef ecosystem. For instance, reduced calcification by corals can reduce the complexity of the coral reef structure, potentially leading to the loss of important habitats for fish and other marine organisms [85]. Furthermore, ocean acidification can impact the symbiotic relationship between corals and their algal symbionts, potentially leading to a decline in the productivity of the reef ecosystem as a whole [86]. The combination of ocean warming and acidification is particularly concerning, as they act synergistically to exacerbate the negative impacts on coral reef ecosystems [22]. With continuing increases in atmospheric CO2 levels, the effects of ocean acidification on coral reefs are expected to become even more pronounced, highlighting the need for urgent action to reduce greenhouse gas emissions and protect these valuable and vulnerable ecosystems.
The rate of atmospheric CO2 increase continues to accelerate, with emission scenarios predicting CO2 concentrations of 540–970 ppm and a decline in seawater pH by 0.14–0.35 units globally for 2100 [80][82]. Fabricius et al. [80] demonstrated that many ecological properties in coral reefs will gradually change as pH declines to 7.8 and that it would be catastrophic for coral reefs if seawater pH dropped below 7.8 (at 750 ppm pCO2).
2.2. Adaptive Strategies for Enhancing Coral Resistance and Resilience in the Face of Climate Change
Coral resistance and resilience are scientific constructs that pertain to the capacity of coral reefs to withstand and recuperate from various stressors. Coral resistance is defined as the ability of corals to endure or tolerate perturbations and stressors, such as variations in water temperature, ocean acidification, pollution, and physical injury. Corals that possess a greater resistance to these stressors exhibit a greater ability to sustain their structure and function despite disturbances and are less prone to suffering from coral bleaching, disease, or mortality [86][87]. A myriad of studies has reported on the bleaching thresholds of corals inhabiting the Persian Gulf, despite conditions at least 2 °C higher than other coral reef ecosystems worldwide [88]. Additionally, corals from the Indo-Pacific and Caribbean regions have been found to maintain calcification rates even in low aragonite saturation states, present in naturally acidified locales [80][89]. The eastern Pacific region of Palau has revealed the thriving of reefs in waters with natural acidification, resulting from biological processes and reef system circulation patterns [89][90]. However, it is noteworthy that coral communities in Palau’s relatively acidic reef zones developed over thousands of years, fostering an inherent resistance that differs from coral communities in regions affected by higher anthropogenic interventions.
Coral resilience, in contrast, refers to the ability of coral reefs to recover from disturbances and stressors. Corals that exhibit higher resilience can reproduce, regenerate, and rebuild their structural complexity after experiencing bleaching [91]. These mechanisms are attributable to genetic diversity within coral populations and their symbiotic association with Symbiodinium algae, which are critical to their health and survival [92][93]. Genetic adaptation in corals is mediated through various factors, including the activation of heat-shock proteins, oxidoreductase enzymes, and microsporine-like amino acids. The coral surface micro-layer that absorbs UV radiation has also been identified as a significant mechanism for adaptation [94][95][96]. In-depth research on corals that thrive in the warm waters of the Persian Gulf has demonstrated their capacity for resilience, attributable to metabolic trade-offs, unique physiological characteristics, and specific genetic signatures, including a heat-specialist algal endosymbiont, Symbiodinium thermophilum [93][97]. S. thermophilum can thrive in high-temperature and high-salinity environments, allowing the coral to develop a temperature-stress-resistant phenotype [97].
Symbiodinium, a diverse group of dinoflagellates, is classified into nine clades (A–I) based on their phylogenetic characteristics [98]. Among these clades, Symbiodinium clade D has garnered attention for its exceptional thermal resilience ability, despite its relatively low representation (less than 10%) in the endosymbiotic community of coral hosts [99]. Various coral species, including fast-growing branching types, such as Acropora, Stylophora, and Pocillopora, as well as slow-growing massive, encrusting, and solitary corals, have been associated with Symbiodinium clade D [100]. The prevalence of clade D Symbiodinium in corals from the Persian Gulf has been linked to their higher thermal tolerance, particularly in comparison to corals associated with clade C, which is the dominant lineage in corals from the Great Barrier Reef and other Pacific coral reef ecosystems [101], and clade B in corals from the Atlantic [102]. These findings highlight the significance of Symbiodinium diversity in understanding the thermal resilience of coral reefs and the potential mechanisms underlying their adaptation to changing environmental conditions.
McCulloch et al. [91] explored the ability of coral species to withstand the adverse impacts of ocean acidification and global warming on coral reefs. Their study revealed that some coral species (i.e., Stylophora pistillata and Porites spp.) exhibit the capacity to increase pH levels within their calcifying fluid, crucial for the deposition of calcium carbonate and maintenance of the coral structure, even in the face of declining seawater pH levels. The study demonstrated the significance of acid-base regulation mechanisms for corals’ resilience to the effects of ocean acidification, allowing them to maintain or increase their calcification rates despite rising ocean acidification. Moreover, the study indicated that corals could acclimate to extended acidification, which enables them to maintain or increase their calcification rates by upregulating their internal pH levels, thus providing insight into potential strategies for mitigating the effects of climate change on coral reefs. A similar adaptation resilience strategy against ocean acidification was observed in cold-water scleractinian corals (i.e., Caryophyllia smithii, Desmophyllum dianthus, Enallopsammia rostrata, Lophelia pertusa, and Madrepora oculate) [103].
Oceanographic processes, such as upwelling and tidal currents, also play a significant role in helping corals avoid bleaching. In areas where upwelling events mix deeper, cooler water with shallow warmer water, thermal stress is reduced [104][105]; for example, in northern Galapagos during the 2015/16 ENSO [106] and Nanwan Bay, southern Taiwan, during summer [107]. Similarly, a coral reef’s ability to resist bleaching is bolstered by the elimination of potentially damaging oxygen radicals due to the swift water flow associated with tidal currents [87][108][109].
This entry is adapted from the peer-reviewed paper 10.3390/ani13050949
References
- Oliver, W.A. Origins and relationships of Paleozoic coral groups and the origin of the Scleractinia. Paleontol. Soc. Pap. 1996, 1, 107–134.
- Spalding, M.D.; Ravilious, C.; Green, E.P. World Atlas of Coral Reefs. Mar. Pollut. Bull. 2002, 44, 350.
- Dishon, G.; Grossowicz, M.; Krom, M.; Guy, G.; Gruber, D.F.; Tchernov, D. Evolutionary Traits that Enable Scleractinian Corals to Survive Mass Extinction Events. Sci. Rep. 2020, 10, 3903.
- Rampino, M.R.; Shen, S.-Z. The end-Guadalupian (259.8 Ma) biodiversity crisis: The sixth major mass extinction? Hist. Biol. 2019, 33, 716–722.
- Antonius, A. Coral reef pathology: A review. In Proceedings of the 4th International Coral Reef Symposium (ICRS), Manila, Phillipines, 27 May—1 June 1985; Volume 1981, pp. 155–160.
- Wilkinson, C.R.; Souter, D. Status of Caribbean Coral Reefs after Bleaching and Hurricanes in 2005; National Oceanic and Atmospheric Administration: Washington, DC, USA, 2008.
- Chung, F.C.; Komilus, C.F.; Mustafa, S. Effect of the creation of a marine protected area on populations of Coral Trout in the coral triangle region. Reg. Stud. Mar. Sci. 2017, 10, 1–9.
- Kamil, T.T.M.; Jamal, A.A.; Fadzli, S.A.; Lananan, F.; Abd Ghani, A.F. Coral Reefs 3D Mapping using Low Cost Autonomous Water Surface Vehicle. Int. J. Appl. Eng. Res. 2017, 12, 14466–14470.
- Burt, J.A.; Camp, E.F.; Enochs, I.C.; Johansen, J.L.; Morgan, K.M.; Riegl, B.; Hoey, A.S. Insights from extreme coral reefs in a changing world. Coral Reefs 2020, 39, 495–507.
- Mohammed, J.S. Applications of 3D printing technologies in oceanography. Methods Oceanogr. 2016, 17, 97–117.
- Wake, D.B.; Vredenburg, V.T. Colloquium paper: Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proc. Natl. Acad. Sci. USA 2008, 105, 11466–11473.
- Barnosky, A.D.; Matzke, N.; Tomiya, S.; Wogan, G.O.U.; Swartz, B.; Quental, T.B.; Marshall, C.; McGuire, J.L.; Lindsey, E.L.; Maguire, K.C.; et al. Has the Earth’s sixth mass extinction already arrived? Nature 2011, 471, 51–57.
- McCallum, M.L. Vertebrate biodiversity losses point to a sixth mass extinction. Biodivers. Conserv. 2015, 24, 2497–2519.
- RCT. The Evolutionary Survival of Coral Reefs. Available online: https://conservation.reefcause.com/the-evolutionary-survival-of-coral-reefs/ (accessed on 21 November 2022).
- Cahill, A.E.; Aiello-Lammens, M.E.; Fisher-Reid, M.C.; Hua, X.; Karanewsky, C.J.; Ryu, H.Y.; Sbeglia, G.C.; Spagnolo, F.; Waldron, J.B.; Warsi, O.; et al. How does climate change cause extinction? Proc. Biol. Sci. 2013, 280, 20121890.
- Munstermann, M.J.; Heim, N.A.; McCauley, D.J.; Payne, J.L.; Upham, N.S.; Wang, S.C.; Knope, M.L. A global ecological signal of extinction risk in terrestrial vertebrates. Conserv. Biol. 2022, 36, e13852.
- Spalding, C.; Hull, P.M. Towards quantifying the mass extinction debt of the Anthropocene. Proc. R. Soc. B Biol. Sci. 2021, 288, 20202332.
- Da Silva, L.B.; Oliveira, G.L.; Frederico, R.G.; Loyola, R.; Zacarias, D.; Ribeiro, B.R.; Mendes-Oliveira, A.C. How future climate change and deforestation can drastically affect the species of monkeys endemic to the eastern Amazon, and priorities for conservation. Biodivers. Conserv. 2022, 31, 971–988.
- Kleypas, J.; Allemand, D.; Anthony, K.; Baker, A.C.; Beck, M.W.; Hale, L.Z.; Hilmi, N.; Hoegh-Guldberg, O.; Hughes, T.; Kaufman, L.; et al. Designing a blueprint for coral reef survival. Biol. Conserv. 2021, 257, 109107.
- Pandolfi, J.M.; Connolly, S.R.; Marshall, D.J.; Cohen, A.L. Projecting Coral Reef Futures Under Global Warming and Ocean Acidification. Science 2011, 333, 418–422.
- Keegan, L.; White, R.; Macinnis-Ng, C. Current knowledge and potential impacts of climate change on New Zealand’s biological heritage. N. Z. J. Ecol. 2022, 46, 1–24.
- Hoegh-Guldberg, O.; Mumby, P.J.; Hooten, A.J.; Steneck, R.S.; Greenfield, P.; Gomez, E.; Harvell, C.D.; Sale, P.F.; Edwards, A.J.; Caldeira, K.; et al. Coral Reefs Under Rapid Climate Change and Ocean Acidification. Science 2007, 318, 1737–1742.
- Hughes, T.P.; Kerry, J.T.; Álvarez-Noriega, M.; Álvarez-Romero, J.G.; Anderson, K.D.; Baird, A.H.; Babcock, R.C.; Beger, M.; Bellwood, D.R.; Berkelmans, R.; et al. Global warming and recurrent mass bleaching of corals. Nature 2017, 543, 373–377.
- Rull, V. Biodiversity crisis or sixth mass extinction? Does the current anthropogenic biodiversity crisis really qualify as a mass extinction? Does the current anthropogenic biodiversity crisis really qualify as a mass extinction? EMBO Rep. 2022, 23, e54193.
- Cowie, R.H.; Bouchet, P.; Fontaine, B. The Sixth Mass Extinction: Fact, fiction or speculation? Biol. Rev. Camb. Philos. Soc. 2022, 97, 640–663.
- Rice, M.M.; Ezzat, L.; Burkepile, D.E. Corallivory in the Anthropocene: Interactive Effects of Anthropogenic Stressors and Corallivory on Coral Reefs. Front. Mar. Sci. 2019, 5, 525.
- Clementi, G.M.; Babcock, E.A.; Valentin-Albanese, J.; Bond, M.E.; Flowers, K.I.; Heithaus, M.R.; Whitman, E.R.; Van Zinnicq Bergmann, M.P.M.; Guttridge, T.L.; O’Shea, O.R.; et al. Anthropogenic pressures on reef-associated sharks in jurisdictions with and without directed shark fishing. Mar. Ecol. Prog. Ser. 2021, 661, 175–186.
- Hughes, T.P.; Anderson, K.D.; Connolly, S.R.; Heron, S.F.; Kerry, J.T.; Lough, J.M.; Baird, A.H.; Baum, J.K.; Berumen, M.L.; Bridge, T.C.; et al. Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 2018, 359, 80–83.
- Van Vuuren, D.P.; Riahi, K.; Calvin, K.; Dellink, R.; Emmerling, J.; Fujimori, S.; Kc, S.; Kriegler, E.; O’Neill, B. The Shared Socio-economic Pathways: Trajectories for human development and global environmental change. Glob. Environ. Chang. 2017, 42, 148–152.
- Cheng, L.; Abraham, J.; Hausfather, Z.; Trenberth, K.E. How fast are the oceans warming? Science 2019, 363, 128–129.
- Doney, S.C.; Fabry, V.J.; Feely, R.A.; Kleypas, J.A. Ocean Acidification: The Other CO2 Problem. Annu. Rev. Mar. Sci. 2009, 1, 169–192.
- Cornwall, C.E.; Comeau, S.; Kornder, N.A.; Perry, C.T.; van Hooidonk, R.; DeCarlo, T.M.; Pratchett, M.S.; Anderson, K.D.; Browne, N.; Carpenter, R.; et al. Global declines in coral reef calcium carbonate production under ocean acidification and warming. Biol. Sci. 2021, 118, e2015265118.
- Riebesell, U.; Gattuso, J.-P. Lessons learned from ocean acidification research. Nat. Clim. Chang. 2014, 5, 12–14.
- Goreau, T.J.; Hayes, R.L. Global change in ocean circulation from satellite sea surface temperature records: Implications for the future of coral-reefs, fisheries, and climate change. In Proceedings of the Oceans 2003. Celebrating the Past, Teaming Toward the Future (IEEE Cat. No.03CH37492), San Diego, CA, USA, 22–26 September 2003; p. 754.
- Dietzel, A.; Connolly, S.R.; Hughes, T.P.; Bode, M. The spatial footprint and patchiness of large-scale disturbances on coral reefs. Glob. Chang. Biol. 2021, 27, 4825–4838.
- Baird, M.E.; Mongin, M.; Skerratt, J.; Margvelashvili, N.; Tickell, S.; Steven, A.D.; Robillot, C.; Ellis, R.; Waters, D.; Kaniewska, P.; et al. Impact of catchment-derived nutrients and sediments on marine water quality on the Great Barrier Reef: An application of the eReefs marine modelling system. Mar. Pollut. Bull. 2021, 167, 112297.
- Kjerfve, B.; McField, M.; Thattai, D.; Giró, A. Coral reef health in the Gulf of Honduras in relation to fluvial runoff, hurricanes, and fishing pressure. Mar. Pollut. Bull. 2021, 172, 112865.
- Tay, C.; Lindsey, E.O.; Chin, S.T.; McCaughey, J.W.; Bekaert, D.; Nguyen, M.; Hua, H.; Manipon, G.; Karim, M.; Horton, B.P.; et al. Sea-level rise from land subsidence in major coastal cities. Nat. Sustain. 2022, 5, 1049–1057.
- Field, M.E.; Ogston, A.S.; Storlazzi, C.D. Rising sea level may cause decline of fringing coral reefs. Eos Trans. Am. Geophys. Union 2011, 92, 273–274.
- Woodroffe, C.D.; Webster, J.M. Coral reefs and sea-level change. Mar. Geol. 2014, 352, 248–267.
- Godoy, M.D.P.; Lacerda, L.D.d. Mangroves Response to Climate Change: A Review of Recent Findings on Mangrove Extension and Distribution. An. Da Acad. Bras. De Ciências 2015, 87, 651–667.
- Rodrigues, E.; Cohen, M.C.L.; Liu, K.-b.; Pessenda, L.C.R.; Yao, Q.; Ryu, J.; Rossetti, D.; de Souza, A.; Dietz, M. The effect of global warming on the establishment of mangroves in coastal Louisiana during the Holocene. Geomorphology 2021, 381, 107648.
- Pérez-Rosales, G.; Pichon, M.; Rouzé, H.; Villéger, S.; Torda, G.; Bongaerts, P.; Carlot, J.; Parravicini, V.; Hédouin, L.; Bardout, G.; et al. Mesophotic coral ecosystems of French Polynesia are hotspots of alpha and beta generic diversity for scleractinian assemblages. Divers. Distrib. 2022, 28, 1391–1403.
- IPCC. Climate Chang. 2014: Synthesis Report; Pachauri, R.K., Meyer, L., Eds.; Intergovermental Panel on Climate Change: Geneva, Switzerland, 2015; p. 151.
- Sully, S.; Hodgson, G.; van Woesik, R. Present and future bright and dark spots for coral reefs through climate change. Glob. Chang. Biol. 2022, 28, 4509–4522.
- IPCC. The Ocean and Cryosphere in a Changing Climate; Cambridge University Press: New York, NY, USA, 2022; p. 716.
- Graham, T.; Idechong, N. Reconciling customary and constitutional law. Ocean Coast. Manag. 1998, 40, 143–164.
- Wabnitz, C.C.C.; Cisneros-Montemayor, A.M.; Hanich, Q.; Ota, Y. Ecotourism, climate change and reef fish consumption in Palau: Benefits, trade-offs and adaptation strategies. Mar. Policy 2018, 88, 323–332.
- Barnett, J. Climate Change and Food Security in the Pacific Islands. In Food Security in Small Island States; Connell, J., Lowitt, K., Eds.; Springer: Gateway East, Singapore, 2019; pp. 25–38.
- Greenstein, B.J.; Pandolfi, J.M. Escaping the heat: Range shifts of reef coral taxa in coastal Western Australia. Glob. Chang. Biol. 2007, 14, 513–528.
- Precht, W.F.; Aronson, R.B. Climate flickers and range shifts of reef corals. Front. Ecol. Environ. 2004, 2, 307–314.
- Yamano, H.; Sugihara, K.; Nomura, K. Rapid poleward range expansion of tropical reef corals in response to rising sea surface temperatures. Geophys. Res. Lett. 2011, 38, L04601.
- Lauria, V.; Massi, D.; Fiorentino, F.; Milisenda, G.; Cillari, T. Habitat suitability mapping of the black coral Leiopathes glaberrima to support conservation of vulnerable marine ecosystems. Sci. Rep. 2021, 11, 15661.
- Wood, S.; Paris, C.B.; Ridgwell, A.; Hendy, E.J. Modelling dispersal and connectivity of broadcast spawning corals at the global scale. Glob. Ecol. Biogeogr. 2013, 23, 1–11.
- Van Hooidonk, R.; Maynard, J.A.; Manzello, D.; Planes, S. Opposite latitudinal gradients in projected ocean acidification and bleaching impacts on coral reefs. Glob. Chang. Biol. 2013, 20, 103–112.
- Muir, P.R.; Wallace, C.C.; Done, T.; Aguirre, J.D. Limited scope for latitudinal extension of reef corals. Science 2015, 348, 1135–1138.
- Azra, M.N.; Aaqillah-Amr, M.A.; Ikhwanuddin, M.; Ma, H.; Waiho, K.; Ostrensky, A.; Tavares, C.P.d.S.; Abol-Munafi, A.B. Effects of climate-induced water temperature changes on the life history of brachyuran crabs. Rev. Aquac. 2020, 12, 1211–1216.
- Azra, M.N.; Noor, M.I.M.; Eales, J.; Sung, Y.Y.; Ghaffar, M.A. What evidence exists for the impact of climate change on the physiology and behaviour of important aquaculture marine crustacean species in Asia? A systematic map protocol. Environ. Evid. 2022, 11, 1–8.
- Eakin, C.M.; Liu, G.; Gomez, A.M.; De la Couri, J.L.; Heron, S.F.; Skirving, W.J.; Strong, A.E. Unprecedented three years of global coral bleaching 2014–2017. Sidebar 3.1.(in state of the climate in 2017). Bull. Am. Meteorol. Soc. 2018, 99, S74–S75.
- Authority, G.B.R.M.P. 2016 Coral Bleaching Event on the Great Barrier Reef; Great Barrier Reef Marine Park Authority: Townsville, QLD, Australia, 2017; ISBN 9780995373167.
- Kim, C.J.S.; Roelfsema, C.; Dove, S.; Hoegh-Guldberg, O. The Condition of Four Coral Reefs in Timor-Leste before and after the 2016–2017 Marine Heatwave. Oceans 2022, 3, 147–171.
- Berkelmans, R.; van Oppen, M.J.H. The role of zooxanthellae in the thermal tolerance of corals: A ‘nugget of hope’ for coral reefs in an era of climate change. Proc. Biol. Sci. 2006, 273, 2305–2312.
- Yu, X.; Yu, K.; Huang, W.; Liang, J.; Qin, Z.; Chen, B.; Yao, Q.; Liao, Z. Thermal acclimation increases heat tolerance of the scleractinian coral Acropora pruinosa. Sci. Total Environ. 2020, 733, 139319.
- Jokiel, P.L.; Coles, S.L. Response of Hawaiian and other Indo-Pacific reef corals to elevated temperature. Coral Reefs 1990, 8, 155–162.
- De’ath, G.; Lough, J.M.; Fabricius, K.E. Declining coral calcification on the Great Barrier Reef. Science 2009, 323, 116–119.
- Anthony, K.R. Coral reefs under climate change and ocean acidification: Challenges and opportunities for management and policy. Annu. Rev. Environ. Resour. 2016, 41, 59–81.
- Heron, S.F.; Maynard, J.A.; van Hooidonk, R.; Eakin, C.M. Warming Trends and Bleaching Stress of the World’s Coral Reefs 1985–2012. Sci. Rep. 2016, 6, 38402.
- Lough, J.M.; Anderson, K.D.; Hughes, T.P. Increasing thermal stress for tropical coral reefs: 1871–2017. Sci. Rep. 2018, 8, 6079.
- Rodolfo-Metalpa, R.; Hoogenboom, M.O.; Rottier, C.; Ramos-Esplá, A.; Baker, A.C.; Fine, M.; Ferrier-Pagès, C. Thermally tolerant corals have limited capacity to acclimatize to future warming. Glob. Chang. Biol. 2014, 20, 3036–3049.
- Kavousi, J.; Tavakoli-Kolour, P.; Mohammadizadeh, M.; Bahrami, A.; Barkhordari, A. Mass coral bleaching in the Northern Persian Gulf, 2012. Sci. Mar. 2014, 78, 397–404.
- Camp, E.F.; Schoepf, V.; Mumby, P.J.; Hardtke, L.A.; Rodolfo-Metalpa, R.; Smith, D.J.; Suggett, D.J. The future of coral reefs subject to rapid climate change: Lessons from natural extreme environments. Front. Mar. Sci. 2018, 5, 4.
- Weis, V.M. Cellular mechanisms of Cnidarian bleaching: Stress causes the collapse of symbiosis. J. Exp. Biol. 2008, 211, 3059–3066.
- Krueger, T.; Horwitz, N.; Bodin, J.; Giovani, M.E.; Escrig, S.; Meibom, A.; Fine, M. Common reef-building coral in the Northern Red Sea resistant to elevated temperature and acidification. R. Soc. Open Sci. 2017, 4, 170038.
- Evensen, N.R.; Fine, M.; Perna, G.; Voolstra, C.R.; Barshis, D.J. Remarkably high and consistent tolerance of a Red Sea coral to acute and chronic thermal stress exposures. Limnol. Oceanogr. 2021, 66, 1718–1729.
- Littman, R.; Willis, B.L.; Bourne, D.G. Metagenomic analysis of the coral holobiont during a natural bleaching event on the Great Barrier Reef. Environ. Microbiol. Rep. 2011, 3, 651–660.
- Doni, L.; Oliveri, C.; Lasa, A.; Di Cesare, A.; Petrin, S.; Martinez-Urtaza, J.; Coman, F.; Richardson, A.; Vezzulli, L. Large-scale impact of the 2016 Marine Heatwave on the plankton-associated microbial communities of the Great Barrier Reef (Australia). Mar. Pollut. Bull. 2023, 188, 114685.
- Bruno, J.F.; Selig, E.R.; Casey, K.S.; Page, C.A.; Willis, B.L.; Harvell, C.D.; Sweatman, H.; Melendy, A.M. Thermal stress and coral cover as drivers of coral disease outbreaks. PLoS Biol. 2007, 5, e124.
- Tignat-Perrier, R.; van de Water, J.A.J.M.; Guillemain, D.; Aurelle, D.; Allemand, D.; Ferrier-Pagès, C. The Effect of Thermal Stress on the Physiology and Bacterial Communities of Two Key Mediterranean Gorgonians. Appl. Environ. Microbiol. 2022, 88, e02340-21.
- Hoey, A.S.; Howells, E.; Johansen, J.L.; Hobbs, J.P.A.; Messmer, V.; McCowan, D.M.; Wilson, S.K.; Pratchett, M.S. Recent advances in understanding the effects of climate change on coral reefs. Diversity 2016, 8, 12.
- Fabricius, K.E.; Langdon, C.; Uthicke, S.; Humphrey, C.; Noonan, S.; De’ath, G.; Okazaki, R.; Muehllehner, N.; Glas, M.S.; Lough, J.M. Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations. Nat. Clim. Chang. 2011, 1, 165–169.
- Orr, J.C.; Fabry, V.J.; Aumont, O.; Bopp, L.; Doney, S.C.; Feely, R.A.; Gnanadesikan, A.; Gruber, N.; Ishida, A.; Joos, F.; et al. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 2005, 437, 681–686.
- Feely, R.A.; Doney, S.C.; Cooley, S.R. Ocean acidification: Present conditions and future changes in a high-CO₂ world. Oceanography 2009, 22, 36–47.
- Albright, R.; Langdon, C. Ocean acidification impacts multiple early life history processes of the Caribbean coral Porites astreoides. Glob. Chang. Biol. 2011, 17, 2478–2487.
- Mera, H.; Bourne, D.G. Disentangling causation: Complex roles of coral-associated microorganisms in disease. Environ. Microbiol. 2018, 20, 431–449.
- Ferrari, M.C.; Munday, P.L.; Rummer, J.L.; McCormick, M.I.; Corkill, K.; Watson, S.A.; Allan, B.J.M.; Meekan, M.G.; Chivers, D.P. Interactive effects of ocean acidification and rising sea temperatures alter predation rate and predator selectivity in reef fish communities. Glob. Chang. Biol. 2015, 21, 1848–1855.
- Mora, C.; Wei, C.-L.; Rollo, A.; Amaro, T.; Baco, A.R.; Billett, D.; Bopp, L.; Chen, Q.; Collier, M.; Danovaro, R. Biotic and human vulnerability to projected changes in ocean biogeochemistry over the 21st century. PLoS Biol. 2013, 11, e1001682.
- Folke, C.; Carpenter, S.; Walker, B.; Scheffer, M.; Elmqvist, T.; Gunderson, L.; Holling, C.S. Regime Shifts, Resilience, and Biodiversity in Ecosystem Management. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 557–581.
- Crook, E.D.; Cohen, A.L.; Rebolledo-Vieyra, M.; Hernandez, L.; Paytan, A. Reduced calcification and lack of acclimatization by coral colonies growing in areas of persistent natural acidification. Proc. Natl. Acad. Sci. USA 2013, 110, 11044–11049.
- Shamberger, K.E.; Cohen, A.L.; Golbuu, Y.; McCorkle, D.C.; Lentz, S.J.; Barkley, H.C. Diverse coral communities in naturally acidified waters of a Western Pacific reef. Geophys. Res. Lett. 2014, 41, 499–504.
- Manzello, D.P. Ocean acidification hot spots: Spatiotemporal dynamics of the seawater CO2 system of eastern Pacific coral reefs. Limnol. Oceanogr. 2010, 55, 239–248.
- McCulloch, M.; Trotter, J.; Montagna, P.; Falter, J.; Dunbar, R.; Freiwald, A.; Försterra, G.; Correa, M.L.; Maier, C.; Rüggeberg, A.; et al. Resilience of cold-water scleractinian corals to ocean acidification: Boron isotopic systematics of pH and saturation state up-regulation. Geochim. Et Cosmochim. Acta 2012, 87, 21–34.
- Howells, M.; Hermann, S.; Welsch, M.; Bazilian, M.; Segerström, R.; Alfstad, T.; Gielen, D.; Rogner, H.; Fischer, G.; van Velthuizen, H.; et al. Integrated analysis of climate change, land-use, energy and water strategies. Nat. Clim. Chang. 2013, 3, 621–626.
- Drollet, J.H.; Glaziou, P.; Martin, P.M.V. A study of mucus from the solitary coral Fungia fungites (Scleractinia: Fungiidae) in relation to photobiological UV adaptation. Mar. Biol. 1993, 115, 263–266.
- LaJeunesse, T.C.; Parkinson, J.E.; Gabrielson, P.W.; Jeong, H.J.; Reimer, J.D.; Voolstra, C.R.; Santos, S.R. Systematic Revision of Symbiodiniaceae Highlights the Antiquity and Diversity of Coral Endosymbionts. Curr. Biol. 2018, 28, 2570–2580.
- Weston, A.J.; Dunlap, W.C.; Shick, J.M.; Klueter, A.; Iglic, K.; Vukelic, A.; Starcevic, A.; Ward, M.; Wells, M.L.; Trick, C.G.; et al. A profile of an endosymbiont-enriched fraction of the coral Stylophora pistillata reveals proteins relevant to microbial-host interactions. Mol. Cell. Proteom. MCP 2012, 11, M111.015487.
- Levin, R.A.; Beltran, V.H.; Hill, R.; Kjelleberg, S.; McDougald, D.; Steinberg, P.D.; van Oppen, M.J.H. Sex, Scavengers, and Chaperones: Transcriptome Secrets of Divergent Symbiodinium Thermal Tolerances. Mol. Biol. Evol. 2016, 33, 2201–2215.
- D’Angelo, C.; Hume, B.C.; Burt, J.; Smith, E.G.; Achterberg, E.P.; Wiedenmann, J. Local adaptation constrains the distribution potential of heat-tolerant Symbiodinium from the Persian/Arabian Gulf. ISME J. 2015, 9, 2551–2560.
- Pochon, X.; Gates, R.D. A new Symbiodinium clade (Dinophyceae) from soritid foraminifera in Hawai’i. Mol. Phylogenet. Evol. 2010, 56, 492–497.
- LaJeunesse, T.C.; Pettay, D.T.; Sampayo, E.M.; Phongsuwan, N.; Brown, B.; Obura, D.O.; Fitt, W.K. Long-standing environmental conditions, geographic isolation and host–symbiont specificity influence the relative ecological dominance and genetic diversification of coral endosymbionts in the genus Symbiodinium. J. Biogeogr. 2010, 37, 785–800.
- Stat, M.; Gates, R.D. Clade D Symbiodinium in scleractinian corals: A “nugget” of hope, a selfish opportunist, an ominous sign, or all of the above? J. Mar. Biol. 2011, 2011, 730715.
- Abrego, D.; Van Oppen, M.J.; Willis, B.L. Highly infectious symbiont dominates initial uptake in coral juveniles. Mol. Ecol. 2009, 18, 3518–3531.
- LaJeunesse, T.C.; Loh, W.K.; Van Woesik, R.; Hoegh-Guldberg, O.; Schmidt, G.W.; Fitt, W.K. Low symbiont diversity in southern Great Barrier Reef corals, relative to those of the Caribbean. Limnol. Oceanogr. 2003, 48, 2046–2054.
- McCulloch, M.; Falter, J.; Trotter, J.; Montagna, P. Coral resilience to ocean acidification and global warming through pH up-regulation. Nat. Clim. Chang. 2012, 2, 623–627.
- Chollett, I.; Mumby, P.J.; Cortés, J. Upwelling areas do not guarantee refuge for coral reefs in a warming ocean. Mar. Ecol. Prog. Ser. 2010, 416, 47–56.
- Porter, S.N.; Sink, K.J.; Schleyer, M.H. The Third Global Coral Bleaching Event on the Marginal Coral Reefs of the Southwestern Indian Ocean and Factors That Contribute to Their Resistance and Resilience. Diversity 2021, 13, 464.
- Riegl, B.; Glynn, P.W.; Banks, S.; Keith, I.; Rivera, F.; Vera-Zambrano, M.; D’Angelo, C.; Wiedenmann, J. Heat attenuation and nutrient delivery by localized upwelling avoided coral bleaching mortality in northern Galapagos during 2015/2016 ENSO. Coral Reefs 2019, 38, 773–785.
- Hsu, P.C.; Lee, H.J.; Zheng, Q.; Lai, J.W.; Su, F.C.; Ho, C.R. Tide-Induced Periodic Sea Surface Temperature Drops in the Coral Reef Area of Nanwan Bay, Southern Taiwan. J. Geophys. Res. Ocean. 2020, 125, e2019JC015226.
- Finelli, C.M.; Helmuth, B.S.; Pentcheff, N.D.; Wethey, D.S. Water flow influences oxygen transport and photosynthetic efficiency in corals. Coral Reefs 2006, 25, 47–57.
- Skirving, W.; Guinotte, J. The Sea Surface Temperature Story on the Great Barrier Reef during the Coral Bleaching Event of 1998. In Oceanographic Processes of Coral Reefs; CRC Press: Boca Raton, FL, USA, 2000; pp. 301–313.
This entry is offline, you can click here to edit this entry!
